Abstract
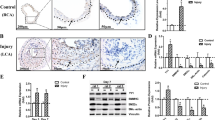
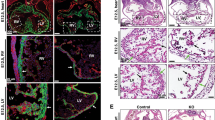
The SET and MYND domain-containing protein 2 (SMYD2) is a histone lysine methyltransferase that has been reported to regulate carcinogenesis and inflammation. However, its role in vascular smooth muscle cell (VSMC) homeostasis and vascular diseases has not been determined. Here, we investigated the role of SMYD2 in VSMC phenotypic modulation and vascular intimal hyperplasia and elucidated the underlying mechanism. We observed that SMYD2 expression was downregulated in injured carotid arteries in mice and phenotypically modulated VSMCs in vitro. Using an SMC-specific SMYD2 knockout mouse model, we found that SMYD2 ablation in VSMCs exacerbated neointima formation after vascular injury in vivo. Conversely, SMYD2 overexpression inhibited VSMC proliferation and migration in vitro and attenuated arterial narrowing in injured vessels in mice. SMYD2 downregulation promoted VSMC phenotypic switching accompanied with enhanced proliferation and migration. Mechanistically, genome-wide transcriptome analysis and loss/gain-of-function studies revealed that SMYD2 up-regulated VSMC contractile gene expression and suppressed VSMC proliferation and migration, in part, by promoting expression and transactivation of the master transcription cofactor myocardin. In addition, myocardin directly interacted with SMYD2, thereby facilitating SMYD2 recruitment to the CArG regions of SMC contractile gene promoters and leading to an open chromatin status around SMC contractile gene promoters via SMYD2-mediated H3K4 methylation. Hence, we conclude that SMYD2 is a novel regulator of VSMC contractile phenotype and intimal hyperplasia via a myocardin-dependent epigenetic regulatory mechanism.









Similar content being viewed by others
Data availability
All data associated with this study are present in the paper or the Supplementary Materials. RNA-seq data are accessible at the GEO under accession number: GSE161004. Any additional information for this study is available by contacting the corresponding authors upon reasonable request.
Abbreviations
- CArG:
-
CC(A/T)6GG
- ChIP:
-
Quantitative chromatin immunoprecipitation
- ChIP-qPCR:
-
Chromatin immunoprecipitation-quantitative PCR
- CT:
-
C-terminal
- H3K36:
-
Histone H3 at lysine 36
- H3K4:
-
Histone H3 at lysine 4
- H4K20:
-
Histone H4 at lysine 20
- HASMC:
-
Human aortic smooth muscle cell
- LCA:
-
Left common carotid artery
- MRTF-A/B:
-
Myocardin-related transcription factor A/B
- PDGF-BB:
-
Platelet-derived growth factor BB
- PLA:
-
Proximity ligation assay
- RCA:
-
Right common carotid artery
- SMYD2:
-
SET and MYND domain-containing protein 2
- SMYD2SMC − / − :
-
SMC-specific SMYD2-deficient mice
- SMYD2fl/fl :
-
SMYD2 homozygous floxed mice
- SRF:
-
Serum response factor
- TAD:
-
Transcription activation domain
- VSMC:
-
Vascular smooth muscle cell
References
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702. https://doi.org/10.1161/CIRCRESAHA.115.306361
Gomez D, Owens GK (2012) Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95:156–164. https://doi.org/10.1093/cvr/cvs115
Alexander MR, Owens GK (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74:13–40. https://doi.org/10.1146/annurev-physiol-012110-142315
Yoshida T et al (2003) Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92:856–864. https://doi.org/10.1161/01.res.0000068405.49081.09
Miano JM, Long X, Fujiwara K (2007) Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292:C70-81. https://doi.org/10.1152/ajpcell.00386.2006
Miano JM (2015) Myocardin in biology and disease. J Biomed Res 29:3–19. https://doi.org/10.7555/jbr.29.20140151
Lockman K, Taylor JM, Mack CP (2007) The histone demethylase, Jmjd1a, interacts with the myocardin factors to regulate SMC differentiation marker gene expression. Circ Res 101:e115-123. https://doi.org/10.1161/circresaha.107.164178
Gan Q et al (2011) WD repeat-containing protein 5, a ubiquitously expressed histone methyltransferase adaptor protein, regulates smooth muscle cell-selective gene activation through interaction with pituitary homeobox 2. J Biol Chem 286:21853–21864. https://doi.org/10.1074/jbc.M111.233098
Liu R et al (2013) Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128:2047–2057. https://doi.org/10.1161/CIRCULATIONAHA.113.002887
Barrero MJ, Boue S, Izpisua Belmonte JC (2010) Epigenetic mechanisms that regulate cell identity. Cell Stem Cell 7:565–570. https://doi.org/10.1016/j.stem.2010.10.009
Bauer AJ, Martin KA (2017) Coordinating regulation of gene expression in cardiovascular disease: interactions between chromatin modifiers and transcription factors. Front Cardiovasc Med 4:19. https://doi.org/10.3389/fcvm.2017.00019
Gomez D, Swiatlowska P, Owens GK (2015) Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler Thromb Vasc Biol 35:2508–2516. https://doi.org/10.1161/atvbaha.115.305044
Liu R, Leslie KL, Martin KA (2015) Epigenetic regulation of smooth muscle cell plasticity. Biochem Biophys Acta 1849:448–453. https://doi.org/10.1016/j.bbagrm.2014.06.004
Villeneuve LM et al (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A 105:9047–9052. https://doi.org/10.1073/pnas.0803623105
Ahmed ASI et al (2018) Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc Natl Acad Sci U S A 115:E8660–E8667. https://doi.org/10.1073/pnas.1803725115
Brown MA, Sims RJ 3rd, Gottlieb PD, Tucker PW (2006) Identification and characterization of SMYD2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer 5:26. https://doi.org/10.1186/1476-4598-5-26
Rice M et al (2014) SMYD2 structure and function: a multispecificity protein lysine methyltransferase. J Cytol Mol Biol 1:7
Du SJ, Tan X, Zhang J (2014) SMYD Proteins: Key Regulators in Skeletal and Cardiac Muscle Development and Function. Anatomical Record (Hoboken, N. J.: 2007) 297:1650–1662. https://doi.org/10.1002/ar.22972
Spellmon N, Holcomb J, Trescott L, Sirinupong N, Yang Z (2015) Structure and Function of SET and MYND Domain-Containing Proteins. Int J Mol Sci 16
Tracy C et al (2018) The Smyd family of methyltransferases: role in cardiac and skeletal muscle physiology and pathology. Curr Opin Physiol 1:140–152. https://doi.org/10.1016/j.cophys.2017.10.001
Abu-Farha M et al (2008) The tale of two domains: proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Molecular & cellular proteomics : MCP 7:560–572. https://doi.org/10.1074/mcp.M700271-MCP200
Boehm D et al (2017) SMYD2-Mediated Histone Methylation Contributes to HIV-1 Latency. Cell Host Microbe 21:569-579 e566. https://doi.org/10.1016/j.chom.2017.04.011
Huang J et al (2006) Repression of p53 activity by SMYD2-mediated methylation. Nature 444:629–632. https://doi.org/10.1038/nature05287
Abu-Farha M et al (2011) Proteomic analyses of the SMYD family interactomes identify HSP90 as a novel target for SMYD2. J Mol Cell Biol 3:301–308. https://doi.org/10.1093/jmcb/mjr025
Cho HS et al (2012) RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia (New York, N.Y.) 14:476–486
Zhang X et al (2013) Regulation of estrogen receptor alpha by histone methyltransferase SMYD2-mediated protein methylation. Proc Natl Acad Sci USA 110:17284–17289. https://doi.org/10.1073/pnas.1307959110
Piao L et al (2014) The histone methyltransferase SMYD2 methylates PARP1 and promotes poly(ADP-ribosyl)ation activity in cancer cells. Neoplasia 16:257–264. https://doi.org/10.1016/j.neo.2014.03.002. (264.e252)
Zeng Y et al (2019) Regulation of EZH2 by SMYD2-Mediated Lysine Methylation Is Implicated in Tumorigenesis. Cell Rep 29:1482-1498 e1484. https://doi.org/10.1016/j.celrep.2019.10.004
Li LX et al (2017) Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J Clin Investig 127:2751–2764. https://doi.org/10.1172/JCI90921
Li LX et al (2018) Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis 9:326. https://doi.org/10.1038/s41419-018-0347-x
Yi X, Jiang XJ, Fang ZM (2019) Histone methyltransferase SMYD2: ubiquitous regulator of disease. Clin Epigenetics 11:112. https://doi.org/10.1186/s13148-019-0711-4
Diehl F et al (2010) Cardiac deletion of SMYD2 is dispensable for mouse heart development. PLoS ONE 5:e9748. https://doi.org/10.1371/journal.pone.0009748
Voelkel T et al (2013) Lysine methyltransferase SMYD2 regulates Hsp90-mediated protection of the sarcomeric titin springs and cardiac function. Biochem Biophys Acta 1833:812–822. https://doi.org/10.1016/j.bbamcr.2012.09.012
Xu G et al (2015) The histone methyltransferase SMYD2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) production. J Biol Chem 290:5414–5423. https://doi.org/10.1074/jbc.M114.610345
Jones GT et al (2017) Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res 120:341–353. https://doi.org/10.1161/CIRCRESAHA.116.308765
Toghill BJ, Saratzis A, Freeman PJ, Sylvius N, Bown MJ (2018) SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells. Clin Epigenetics 10:29. https://doi.org/10.1186/s13148-018-0460-9
Skarnes WC et al (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474:337–342. https://doi.org/10.1038/nature10163
Holtwick R et al (2002) Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 99:7142–7147. https://doi.org/10.1073/pnas.102650499
Hu G et al (2010) Modulation of myocardin function by the ubiquitin E3 ligase UBR5. J Biol Chem 285:11800–11809. https://doi.org/10.1074/jbc.M109.079384
Wang X et al (2012) The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler Thromb Vasc Biol 32:2662–2669. https://doi.org/10.1161/atvbaha.112.254730
Osman I et al (2019) TEAD1 (TEA Domain Transcription Factor 1) Promotes Smooth Muscle Cell Proliferation Through Upregulating SLC1A5 (Solute Carrier Family 1 Member 5)-Mediated Glutamine Uptake. Circ Res 124:1309–1322. https://doi.org/10.1161/CIRCRESAHA.118.314187
Xu F et al (2015) MicroRNA-15b/16 attenuates vascular neointima formation by promoting the contractile phenotype of vascular smooth muscle through targeting YAP. Arterioscler Thromb Vasc Biol 35:2145–2152. https://doi.org/10.1161/atvbaha.115.305748
Wen T et al (2019) Transcription factor TEAD1 is essential for vascular development by promoting vascular smooth muscle differentiation. Cell Death Differ 26:2790–2806. https://doi.org/10.1038/s41418-019-0335-4
Rothman A et al (1992) Development and characterization of a cloned rat pulmonary arterial smooth muscle cell line that maintains differentiated properties through multiple subcultures. Circulation 86:1977–1986
Eggert E et al (2016) Discovery and characterization of a highly potent and selective aminopyrazoline-based in vivo probe (BAY-598) for the protein lysine methyltransferase SMYD2. J Med Chem 59:4578–4600. https://doi.org/10.1021/acs.jmedchem.5b01890
Shang L, Wei M (2019) Inhibition of SMYD2 sensitized cisplatin to resistant cells in NSCLC through activating p53 pathway. Front Oncol 9:306. https://doi.org/10.3389/fonc.2019.00306
Ran FA et al (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. https://doi.org/10.1038/nprot.2013.143
Zhou Y et al (2019) LncRNA CRNDE regulates the proliferation and migration of vascular smooth muscle cells. J Cell Physiol 234:16205–16214. https://doi.org/10.1002/jcp.28284
Farooq SM et al (2018) Disruption of GPR35 exacerbates dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 63:2910–2922. https://doi.org/10.1007/s10620-018-5216-z
Li C et al (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131:940–951. https://doi.org/10.1016/j.cell.2007.09.037
Hou Y et al (2015) A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem cell research 14:133–143. https://doi.org/10.1016/j.scr.2014.12.001
Wu Y et al (2012) A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem 287:5744–5755. https://doi.org/10.1074/jbc.M111.315762
Zhou J, Blue EK, Hu G, Herring BP (2008) Thymine DNA glycosylase represses myocardin-induced smooth muscle cell differentiation by competing with serum response factor for myocardin binding. J Biol Chem 283:35383–35392. https://doi.org/10.1074/jbc.M805489200
Salmon M, Gomez D, Greene E, Shankman L, Owens GK (2012) Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22alpha promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res 111:685–696. https://doi.org/10.1161/circresaha.112.269811
Nagao M et al (2020) Coronary disease-associated gene TCF21 Inhibits smooth muscle cell differentiation by blocking the myocardin-serum response factor pathway. Circ Res 126:517–529. https://doi.org/10.1161/CIRCRESAHA.119.315968
Liu J et al (2021) TEAD1 protects against necroptosis in postmitotic cardiomyocytes through regulation of nuclear DNA-encoded mitochondrial genes. Cell Death Differ. https://doi.org/10.1038/s41418-020-00732-5
Mack CP, Owens GK (1999) Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5’ and first intron promoter regions. Circ Res 84:852–861
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801
Kumar A, Lindner V (1997) Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17:2238–2244. https://doi.org/10.1161/01.atv.17.10.2238
Yang F et al (2018) miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 137:1824–1841. https://doi.org/10.1161/circulationaha.117.027799
Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK (1992) Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 71:1525–1532
Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW (1992) Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Investig 89:507–511. https://doi.org/10.1172/JCI115613
Cao D et al (2005) Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol 25:364–376. https://doi.org/10.1128/mcb.25.1.364-376.2005
Spellmon N, Holcomb J, Trescott L, Sirinupong N, Yang Z (2015) Structure and function of SET and MYND domain-containing proteins. Int J Mol Sci 16:1406–1428. https://doi.org/10.3390/ijms16011406
Talasila A et al (2013) Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-beta. Arterioscler Thromb Vasc Biol 33:2355–2365. https://doi.org/10.1161/atvbaha.112.301000
Ackers-Johnson M et al (2015) Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arterioscler Thromb Vasc Biol 35:817–828. https://doi.org/10.1161/atvbaha.114.305218
Ueyama T, Kasahara H, Ishiwata T, Nie Q, Izumo S (2003) Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol Cell Biol 23:9222–9232. https://doi.org/10.1128/mcb.23.24.9222-9232.2003
Ruthenburg AJ, Allis CD, Wysocka J (2007) Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell 25:15–30. https://doi.org/10.1016/j.molcel.2006.12.014
Wang Z et al (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138:1019–1031. https://doi.org/10.1016/j.cell.2009.06.049
McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK (2006) Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Investig 116:36–48. https://doi.org/10.1172/jci26505
Hu M, Hiroyasu S, Granville DJ, Kassiri Z (2022) Implications of Sm22alpha-Cre expression in keratinocytes and unanticipated inflammatory skin lesion in a model of atherosclerosis. Am J Physiol Heart Circ Physiol 323:H528–H534. https://doi.org/10.1152/ajpheart.00325.2022
Chakraborty R et al (2019) Promoters to study vascular smooth muscle. Arterioscler Thromb Vasc Biol 39:603–612. https://doi.org/10.1161/ATVBAHA.119.312449
Warthi G et al (2022) Generation and comparative analysis of an Itga8-CreER (T2) mouse with preferential activity in vascular smooth muscle cells. Nat Cardiovasc Res 1:1084–1100. https://doi.org/10.1038/s44161-022-00162-1
Yang D et al (2021) H3K4 methyltransferase Smyd3 mediates vascular smooth muscle cell proliferation, migration, and neointima formation. Arterioscler Thromb Vasc Biol 41:1901–1914. https://doi.org/10.1161/ATVBAHA.121.314689
Acknowledgements
We thank Dr. Chengliu Jin and Zoe Liu (Transgenic Core at GSU) for generous help with generating the SMYD2 KO mice.
Funding
This work was supported by startup fund from Georgia State University (to C Li) and by grants from National Natural Science Foundation of China (82000448 to Y Zhou) and from National Institutes of Health (HL128647 to C Li, HL149995 to J Zhou, and HL140954 to P Song and M-H Zou). C Li is a recipient of Transformational Project Award (20TPA35410012) from American Heart Association. S. Sharma is a recipient of Pre-doctoral Fellowship (917128) from American Heart Association. J Zhou is a recipient of Established Investigator Award (17EIA33460468) and Transformational Project Award (19TPA34910181) from American Heart Association. M-H Zou is a Georgia Research Alliance Eminent Scholar in Molecular Medicine.
Author information
Authors and Affiliations
Contributions
YZ designed and performed most of the experiments and analyzed data. SS and XS performed some data collection. CL and ZH conceived, designed, and supervised the study. YZ and CL wrote and revised the manuscript. JZ and PS provided reagents and revised the manuscript. XG, YH, ZY, HS, M-HZ, PS, JZ, and SW contributed to discussion and interpretation of the data and review of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no potential conflicts of interest.
Ethics approval
All animal protocols were approved by the Institutional Animal Care and Use Committee at Georgia State University in accordance with NIH guidelines.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Sharma, S., Sun, X. et al. SMYD2 regulates vascular smooth muscle cell phenotypic switching and intimal hyperplasia via interaction with myocardin. Cell. Mol. Life Sci. 80, 264 (2023). https://doi.org/10.1007/s00018-023-04883-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04883-9