Abstract
Background
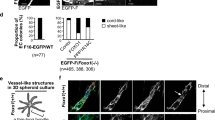
We have shown that Hippo-YAP signaling pathway plays an important role in endothelial cell differentiation. Vestigial-like family member 4 (VGLL4) has been identified as a YAP inhibitor. However, the exact function of VGLL4 in vascular endothelial cell development remains unclear. In this study, we investigated the role of VGLL4, in human endothelial lineage specification both in 3D vascular organoid and 2D endothelial cell differentiation.
Methods and results
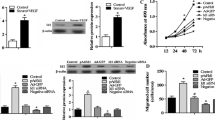
In this study, we found that VGLL4 was increased during 3D vascular organoids generation and directed differentiation of human embryonic stem cells H1 towards the endothelial lineage. Using inducible ectopic expression of VGLL4 based on the piggyBac system, we proved that overexpression of VGLL4 in H1 promoted vascular organoids generation and endothelial cells differentiation. In contrast, VGLL4 knockdown (heterozygous knockout) of H1 exhibited inhibitory effects. Using bioinformatics analysis and protein immunoprecipitation, we further found that VGLL4 binds to TEAD1 and facilitates the expression of endothelial master transcription factors, including FLI1, to promote endothelial lineage specification. Moreover, TEAD1 overexpression rescued VGLL4 knockdown-mediated negative effects.
Conclusions
In summary, VGLL4 promotes EC lineage specification both in 3D vascular organoid and 2D EC differentiation from pluripotent stem cell, VGLL4 interacts with TEAD1 and facilitates EC key transcription factor, including FLI1, to enhance EC lineage specification.







Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jun W, Shijie L, Todd H, Martin JF (2018) The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. https://doi.org/10.1038/s41569-018-0063-3
Randy J, Georg H (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. https://doi.org/10.1038/nrd4161
Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z (2014) VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res 24:331–343
Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN (2011) Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 4:ra70–ra70
von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD (2012) YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci 109:2394–2399
Wang X, Valls AF, Schermann G, Shen Y, Moya IM, Castro L, Urban S, Solecki GM, Winkler F, Riedemann L (2017) YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell 42(462–478):e7
Chen Z, Friedrich GA, Soriano P (1994) Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev 8:2293–2301
He J, Bao Q, Yan M, Liang J, Zhu Y, Wang C, Ai D (2018) The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br J Pharmacol 175:1354–1361
Cho H, Kim J, Ahn JH, Hong Y-K, Mäkinen T, Lim D-S, Koh GY (2019) YAP and TAZ negatively regulate Prox1 during developmental and pathologic lymphangiogenesis. Circ Res 124:225–242
Quan Y, Shan X, Hu M, Jin P, Ma J, Fan J, Yang J, Zhang H, Fan X, Gong Y (2022) YAP inhibition promotes endothelial cell differentiation from pluripotent stem cell through EC master transcription factor FLI1. J Mol Cell Cardiol 163:81–96
Deng X, Fang L (2018) VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am J Cancer Res 8:932
Suo J, Feng X, Li J (2020) VGLL4 promotes osteoblast differentiation by antagonizing TEADs-inhibited Runx2 transcription. Sci Adv 6:4eaba4147
Feng X, Wang Z, Wang F, Lu T, Xu J, Ma X, Li J, He L, Zhang W, Li S (2019) Dual function of VGLL 4 in muscle regeneration. EMBO J 38:e101051
Yu W, Ma X, Xu J, Heumüller AW, Fei Z, Feng X, Wang X, Liu K, Li J, Cui G (2019) VGLL4 plays a critical role in heart valve development and homeostasis. PLoS Genet 15:e1007977
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10:1–22
Wernly B, Mirna M, Rezar R, Prodinger C, Jung C, Podesser BK, Kiss A, Hoppe UC, Lichtenauer M (2019) Regenerative cardiovascular therapies: stem cells and beyond. Int J Mol Sci 20:1420
Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA (2019) Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565:505–510
Wimmer RA, Leopoldi A, Aichinger M, Kerjaschki D, Penninger JM (2019) Generation of blood vessel organoids from human pluripotent stem cells. Nat Protoc 14:3082–3100
Quan Y, Zhang T, Zhang H, Yang J, Wang Y (2020) Generation of a human embryonic stem cell (WAe001-A-47) with hVGLL4 doxycyclin-inducible expression by the PiggyBac transposon system. Stem Cell Res 50:102142
Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K (2015) Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol 17:994–1003
Moya IM, Halder G (2019) Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol 20:211–226
Zhang Y, Shen H, Withers HG, Yang N, Denson KE, Mussell AL, Truskinovsky A, Fan Q, Gelman IH, Frangou C (2017) VGLL4 selectively represses YAP-dependent gene induction and tumorigenic phenotypes in breast cancer. Sci Rep 7:6190
Chen R, Liu Y, Zhuang H, Yang B, Hei K, Xiao M, Hou C, Gao H, Zhang X, Jia C (2017) Quantitative proteomics reveals that long non-coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res 45:9947–9959
Han X-Y, Liu H-Z, Cai C-Y, Li X-Q, Ju Y-B, Li Y-Y, Zhang Z-G (2018) Expression of VGLL4 and YAP protein in gastric carcinoma tissues and tumor prognosis. Minerva Med 109:429–435
Tajonar A, Maehr R, Hu G, Sneddon JB, Rivera-Feliciano J, Cohen DE, Elledge SJ, Melton DA (2013) Brief report: VGLL4 is a novel regulator of survival in human embryonic stem cells. Stem Cells 31:2833–2841
Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, Vandusen N, Guo Y, Zhang J, Stevens SM (2016) Acetylation of VGLL4 regulates Hippo-YAP signaling and postnatal cardiac growth. Dev Cell 39:466-479
Estarás C, Benner C, Jones KA (2015) SMADs and YAP compete to control elongation of β-catenin:LEF-1-recruited RNAPII during hESC differentiation. Mol Cell 58:780–793
Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L, Zhou Z (2017) VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. https://doi.org/10.1038/ncomms14058
Zhang W, Xu J, Li J, Guo T, Jiang D, Feng X, Ma X, He L, Wu W, Yin M (2018) The TEA domain family transcription factor TEAD4 represses murine adipogenesis by recruiting the cofactors VGLL4 and CtBP2 into a transcriptional complex. J Biol Chem 293:17119–17134
Teng AC, Kuraitis D, Deeke SA, Ahmadi A, Dugan SG, Cheng BL, Crowson MG, Burgon PG, Suuronen EJ, Chen HH, Stewart AF (2010) IRF2BP2 is a skeletal and cardiac muscle-enriched ischemia-inducible activator of VEGFA expression. FASEB J 24(12):4825-4834
Wang Y, Liu X, Xie B, Yuan H, Zhang Y, Zhu J (2020) The NOTCH1-dependent HIF1α/VGLL4/IRF2BP2 oxygen sensing pathway triggers erythropoiesis terminal differentiation. Redox Biol 28:101313
Wang Z, Quan Y, Hu M, Xu Y, Chen Y, Jin P, Ma J, Chen X, Fan J, Fan X, Gong Y, Li M, Wang Y (2023) VGLL4-TEAD1 promotes vascular smooth muscle cell differentiation from human pluripotent stem cells via TET2. J Mol Cell Cardiol 176:21–32
Acknowledgements
We would like to thank all members of the Institute of Hypoxia Medicine who contributed to this work. We appreciate the great help of Dr. Lei Zhang (Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences), Dr. Jiaxi Zhou (State Key Laboratory of Experimental Hematology in China), Dr. Bin Zhao (Life Sciences Institute and Innovation Center for Cell Signaling Network, Zhejiang University), and Faxing Yu (School of Life Sciences, Fudan University) for this project.
Funding
This work was supported by the National Key Research and Development Program of China (2019YFA0802702), National Natural Science Foundation of China (82070487, 81670454, and 32070804), Natural Science Foundation of Zhejiang Province (LY21C120003), and Scientific Research Start-up Fund of Wenzhou Medical University (QTJ15029).
Author information
Authors and Affiliations
Contributions
YW and YY contributed to the conception, design, and financial support of the study. YQ, MH, and JJ performed the experiments, analyzed and interpreted the data. JM, ML, JF, XF, and YG assisted with experiments. YW and YQ analyzed the data and wrote the manuscript. YW and YY edited the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and Consent to participate
This study was approved by the Medical Research Ethics Committee of Wenzhou Medical University (No.2019–214). The investigation conformed to the principles outlined in the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quan, Y., Hu, M., Jiang, J. et al. VGLL4 promotes vascular endothelium specification via TEAD1 in the vascular organoids and human pluripotent stem cells-derived endothelium model. Cell. Mol. Life Sci. 80, 215 (2023). https://doi.org/10.1007/s00018-023-04858-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04858-w