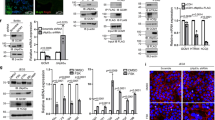
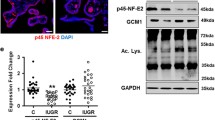
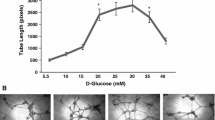
Abstract
Deficiency of decidual NK (dNK) cell number and function has been widely regarded as an important cause of spontaneous abortion. However, the metabolic mechanism underlying the crosstalk between dNK cells and embryonic trophoblasts during early pregnancy remains largely unknown. Here, we observed that enriched glutamine and activated glutaminolysis in dNK cells contribute to trophoblast invasion and embryo growth by insulin-like growth factor-1 (IGF-1) and growth differentiation factor-15 (GDF-15) secretion. Mechanistically, these processes are dependent on the downregulation of EGLN1-HIF-1α mediated by α-ketoglutarate (α-KG). Blocking glutaminolysis with the GLS inhibitor BPTES or the glutamate dehydrogenase inhibitor EGCG leads to early embryo implantation failure, spontaneous abortion and/or fetal growth restriction in pregnant mice with impaired trophoblast invasion. Additionally, α-KG supplementation significantly alleviated pregnancy loss mediated by defective glutaminolysis in vivo, suggesting that inactivated glutamine/α-ketoglutarate metabolism in dNK cells impaired trophoblast invasion and induced pregnancy loss.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Abbreviations
- α-KG:
-
α-Ketoglutarate
- ACTB:
-
β-Actin
- BPTES:
-
Inhibitor of glutaminase
- dNK:
-
Decidual NK cells
- DIC:
-
Decidual immune cells
- DMKG:
-
Dimethyl alpha-ketoglutarate
- DMOG:
-
Dimethyloxallyl glycine, inhibitor of HIF prolyl-hydroxylase
- DSC:
-
Decidual stromal cells
- eNK:
-
Endometrial NK cells
- EGCG:
-
Epigallocatechin gallate sulfate, inhibitor of glutamate dehydrogenase
- EGLN1:
-
Egl nine homolog 1
- EP300:
-
E1A binding protein p300
- ESC:
-
Endometrial stromal cells
- ESR1:
-
Estrogen receptor 1
- FGF-19:
-
Fibroblast growth factor-19
- GABA:
-
γ-Aminobutyric acid
- GDF15:
-
Growth differentiation factor-15
- GLS:
-
Glutaminase
- GLUD:
-
Glutamate dehydrogenase
- HIF-1α:
-
Hypoxia inducible factor-1α
- IGF1:
-
Insulin-like growth factor-1
- IUGR:
-
Intrauterine growth restriction
- OXPHOS:
-
Oxidative phosphorylation
- PBMC:
-
Peripheral blood mononuclear cells
- pNK:
-
Peripheral NK cells
- TCA:
-
Tricarboxylic acid
- TET:
-
Ten-eleven translocation methylcytosine dioxygenase
References
Musallam R, Salem N, Al Halol R, Al Deeb H, Bottcher B, AlHamaida H (2018) Management of pregnancy loss in the first trimester: a retrospective audit. Lancet (London, England) 391(Suppl 2):S34. https://doi.org/10.1016/S0140-6736(18)30400-8
Garrido-Gimenez C, Alijotas-Reig J (2015) Recurrent miscarriage: causes, evaluation and management. Postgrad Med J 91:151–162. https://doi.org/10.1136/postgradmedj-2014-132672
Gellersen B, Brosens JJ (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 35:851–905. https://doi.org/10.1210/er.2014-1045
Tao Y, Li Y-H, Piao H-L, Zhou W-J, Zhang D, Fu Q et al (2015) CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol Immunol 12:77–86. https://doi.org/10.1038/cmi.2014.26
Keskin DB, Allan DSJ, Rybalov B, Andzelm MM, Stern JNH, Kopcow HD et al (2007) TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 104:3378–3383
Jabrane-Ferrat N (2019) Features of human decidual NK cells in healthy pregnancy and during viral infection. Front Immunol 10:1397. https://doi.org/10.3389/fimmu.2019.01397
Erlebacher A (2013) Immunology of the maternal–fetal interface. Annu Rev Immunol 31:387–411. https://doi.org/10.1146/annurev-immunol-032712-100003
Zhou Y, Fu B, Xu X, Zhang J, Tong X, Wang Y et al (2020) PBX1 expression in uterine natural killer cells drives fetal growth. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax1798
Lu H, Yang H-L, Zhou W-J, Lai Z-Z, Qiu X-M, Fu Q et al (2020) Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence. Autophagy. https://doi.org/10.1080/15548627.2020.1833515
Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z et al (2017) Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. https://doi.org/10.1016/j.immuni.2017.11.018
Cartwright JE, James-Allan L, Buckley RJ, Wallace AE (2017) The role of decidual NK cells in pregnancies with impaired vascular remodelling. J Reprod Immunol 119:81–84. https://doi.org/10.1016/j.jri.2016.09.002
Seshadri S, Sunkara SK (2014) Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update 20:429–438. https://doi.org/10.1093/humupd/dmt056
Wang F, Jia W, Fan M, Shao X, Li Z, Liu Y et al (2021) Single-cell Immune landscape of human recurrent miscarriage. Genom Proteom Bioinform 19:208–222. https://doi.org/10.1016/j.gpb.2020.11.002
Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB et al (2018) Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563:347–353. https://doi.org/10.1038/s41586-018-0698-6
Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A et al (2018) A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 4:eaau4788. https://doi.org/10.1126/sciadv.aau4788
Du L, Deng W, Zeng S, Xu P, Huang L, Liang Y et al (2021) Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Prolif 54:e13125. https://doi.org/10.1111/cpr.13125
Assmann N, O’Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM et al (2017) Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol 18:1197–1206. https://doi.org/10.1038/ni.3838
Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K et al (2018) Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 19:1330–1340. https://doi.org/10.1038/s41590-018-0251-7
Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R et al (2018) Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. https://doi.org/10.1016/j.cmet.2018.06.021
Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL et al (2021) Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. https://doi.org/10.1016/j.cmet.2021.03.023
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P (2018) Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. https://doi.org/10.3390/nu10111564
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR et al (2021) Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593:282–288. https://doi.org/10.1038/s41586-021-03442-1
Leone RD, Zhao L, Englert JM, Sun I-M, Oh M-H, Sun I-H et al (2019) Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science (New York, NY) 366:1013–1021. https://doi.org/10.1126/science.aav2588
Arts RJW, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E et al (2016) Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24:807–819. https://doi.org/10.1016/j.cmet.2016.10.008
Oh M-H, Sun I-H, Zhao L, Leone RD, Sun I-M, Xu W et al (2020) Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Investig 130:3865–3884. https://doi.org/10.1172/JCI131859
Loftus RM, Assmann N, Kedia-Mehta N, O’Brien KL, Garcia A, Gillespie C et al (2018) Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun 9:2341. https://doi.org/10.1038/s41467-018-04719-2
Lu H, Jin L-P, Huang H-L, Ha S-Y, Yang H-L, Chang R-Q et al (2020) Trophoblast-derived CXCL12 promotes CD56 CD82 CD29 NK cell enrichment in the decidua. Am J Reprod Immunol. https://doi.org/10.1111/aji.13203
Yang S-L, Tan H-X, Niu T-T, Li D-J, Wang H-Y, Li M-Q (2021) Kynurenine promotes the cytotoxicity of NK cells through aryl hydrocarbon receptor in early pregnancy. J Reprod Immunol 143:103270. https://doi.org/10.1016/j.jri.2020.103270
Mei J, Zhou W-J, Zhu X-Y, Lu H, Wu K, Yang H-L et al (2018) Suppression of autophagy and HCK signaling promotes PTGS2 FCGR3 NK cell differentiation triggered by ectopic endometrial stromal cells. Autophagy 14:1376–1397. https://doi.org/10.1080/15548627.2018.1476809
Olsen OE, Skjærvik A, Størdal BF, Sundan A, Holien T (2017) TGF-β contamination of purified recombinant GDF15. PLoS ONE 12:e0187349. https://doi.org/10.1371/journal.pone.0187349
Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM et al (2018) Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun 9:4456. https://doi.org/10.1038/s41467-018-06893-9
Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC et al (2018) Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. https://doi.org/10.1016/j.cell.2018.10.001
Nachef M, Ali AK, Almutairi SM, Lee S-H (2021) Targeting SLC1A5 and SLC3A2/SLC7A5 as a potential strategy to strengthen anti-tumor immunity in the tumor microenvironment. Front Immunol 12:624324. https://doi.org/10.3389/fimmu.2021.624324
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH et al (2020) A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. https://doi.org/10.1016/j.cmet.2019.11.020
Pérez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hée VF, De Saedeleer CJ et al (2016) Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell cycle (Georgetown, Tex) 15:72–83. https://doi.org/10.1080/15384101.2015.1120930
Scalise M, Pochini L, Console L, Losso MA, Indiveri C (2018) The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol 6:96. https://doi.org/10.3389/fcell.2018.00096
Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S et al (2012) Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483:484–488. https://doi.org/10.1038/nature10898
Baksh SC, Finley LWS (2021) Metabolic coordination of cell fate by α-ketoglutarate-dependent dioxygenases. Trends Cell Biol 31:24–36. https://doi.org/10.1016/j.tcb.2020.09.010
Rasmussen KD, Helin K (2016) Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30:733–750. https://doi.org/10.1101/gad.276568.115
Ivan M, Kaelin WG (2017) The EGLN-HIF O-sensing system: multiple inputs and feedbacks. Mol Cell 66:772–779. https://doi.org/10.1016/j.molcel.2017.06.002
Crespo ÂC, Mulik S, Dotiwala F, Ansara JA, Sen Santara S, Ingersoll K et al (2020) Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell. https://doi.org/10.1016/j.cell.2020.07.019
Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R et al (2018) Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity. https://doi.org/10.1016/j.immuni.2018.03.030
Chong WP, van Panhuys N, Chen J, Silver PB, Jittayasothorn Y, Mattapallil MJ et al (2015) NK-DC crosstalk controls the autopathogenic Th17 response through an innate IFN-γ-IL-27 axis. J Exp Med 212:1739–1752. https://doi.org/10.1084/jem.20141678
Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D et al (2010) Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA 107:11918–11923. https://doi.org/10.1073/pnas.1001749107
Waters MJ, Brooks AJ (2012) Growth hormone and cell growth. Endocr Dev 23:86–95. https://doi.org/10.1159/000341761
Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL et al (2020) Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab 2:278–289. https://doi.org/10.1038/s42255-020-0184-y
Xu J, Wang X, Chen J, Chen S, Li Z, Liu H et al (2020) Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics 10:12204–12222. https://doi.org/10.7150/thno.47683
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS et al (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113:E7554–E7563
Higashi Y, Sukhanov S, Shai S-Y, Danchuk S, Tang R, Snarski P et al (2016) Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation 133:2263–2278. https://doi.org/10.1161/CIRCULATIONAHA.116.021805
Shi J-W, Yang H-L, Lai Z-Z, Shen H-H, Qin X-Y, Qiu X-M et al (2021) WISP2/IGF1 promotes the survival of DSCs and impairs the cytotoxicity of decidual NK cells. Reproduction 161:425–436. https://doi.org/10.1530/REP-20-0658
Lindsay RS, Hamilton BA, Calder AA, Johnstone FD, Walker JD (2004) The relation of insulin, leptin and IGF-1 to birthweight in offspring of women with type 1 diabetes. Clin Endocrinol (Oxf) 61:353–359
Kelly AC, Powell TL, Jansson T (2020) Placental function in maternal obesity. Clin Sci (Lond, Engl) 134:961–984. https://doi.org/10.1042/CS20190266
Niu Z-R, Han T, Sun X-L, Luan L-X, Gou W-L, Zhu X-M (2018) MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2017.11.568
Wu H-Y, Wang X-H, Liu K, Zhang J-L (2020) LncRNA MALAT1 regulates trophoblast cells migration and invasion via miR-206/IGF-1 axis. Cell cycle (Georgetown, Tex) 19:39–52. https://doi.org/10.1080/15384101.2019.1691787
Wischhusen J, Melero I, Fridman WH (2020) Growth/differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint. Front Immunol 11:951. https://doi.org/10.3389/fimmu.2020.00951
Kleinertz H, Hepner-Schefczyk M, Ehnert S, Claus M, Halbgebauer R, Boller L et al (2019) Circulating growth/differentiation factor 15 is associated with human CD56 natural killer cell dysfunction and nosocomial infection in severe systemic inflammation. EBioMedicine 43:380–391. https://doi.org/10.1016/j.ebiom.2019.04.018
Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN et al (2010) GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res 16:3851–3859. https://doi.org/10.1158/1078-0432.CCR-10-0705
Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET (2019) Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 38:4540–4559. https://doi.org/10.1038/s41388-019-0736-3
Acknowledgements
We are grateful to Prof. Shi-Min Zhao from Institute of Metabolism and Integrative Biology (IMIB), School of Life Sciences, Fudan University, for guidance and help. This study supported by the National Natural Science Foundation of China (NSFC) (No. 92057119, 31970798, 32070915), the National Key Research and Development Program of China (2017YFC1001404), the Program for Zhuoxue of Fudan University (JIF157602), the Support Project for Original Personalized Research of Fudan University, and the Yantai Science and Technology Innovation Plan (2021XDHZ082).
Funding
Funding was provided by National Natural Science Foundation of China (92057119, 31970798, 32070915), National Key Research and Development Program of China (2017YFC1001404), Program for Zhuoxue of Fudan University (JIF157602), Support Project for Original Personalized Research of Fudan University, Yantai Science and Technology Innovation Plan (2021XDHZ082).
Author information
Authors and Affiliations
Contributions
SLY and HXT conducted all experiments and prepared the figures and the manuscript. ZZL, HYP and HLY assisted with cell sorting, in vivo experiments, prepared the figures and the manuscript. QF edited the manuscript. MQL, DJL and HYW initiated and supervised the project and edited the manuscript. All the authors were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and all experiments were approved by The Animal Care and Use Committee of Fudan University.
Consent for publication
All authors consent to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, SL., Tan, HX., Lai, ZZ. et al. An active glutamine/α-ketoglutarate/HIF-1α axis prevents pregnancy loss by triggering decidual IGF1+GDF15+NK cell differentiation. Cell. Mol. Life Sci. 79, 611 (2022). https://doi.org/10.1007/s00018-022-04639-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04639-x