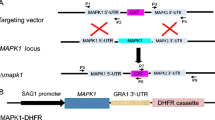
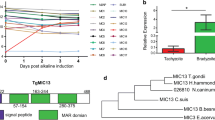
Abstract
Toxoplasma gondii is a widespread eukaryotic pathogen that causes life-threatening diseases in humans and diverse animals. It has a complex life cycle with multiple developmental stages, which are timely adjusted according to growth conditions. But the regulatory mechanisms are largely unknown. Here we show that the AMP-activated protein kinase (AMPK), a key regulator of energy homeostasis in eukaryotes, plays crucial roles in controlling the cell cycle progression and bradyzoite development in Toxoplasma. Deleting the β regulatory subunit of AMPK in the type II strain ME49 caused massive DNA damage and increased spontaneous conversion to bradyzoites (parasites at chronic infection stage), leading to severe growth arrest and reduced virulence of the parasites. Under alkaline stress, all Δampkβ mutants converted to a bradyzoite-like state but the cell division pattern was significantly impaired, resulting in compromised parasite viability. Moreover, we found that phosphorylation of the catalytic subunit AMPKα was greatly increased in alkaline stressed parasites, whereas AMPKβ deletion mutants failed to do so. Phosphoproteomics found that many proteins with predicted roles in cell cycle and cell division regulation were differentially phosphorylated after AMPKβ deletion, under both normal and alkaline stress conditions. Together, these results suggest that the parasite AMPK has critical roles in safeguarding cell cycle progression, and guiding the proper exist of the cell cycle to form mature bradyzoites when the parasites are stressed. Consistent with this model, growth of parasites was not significantly altered when AMPKβ was deleted in a strain that was naturally reluctant to bradyzoite development.








Similar content being viewed by others
Data availability
All data relevant to study are available within this manuscript and its supplementary materials. Phospho-proteomic data generated in this study were deposited in iProx (URL: https://www.iprox.cn/page/DSV021.html;?url=1650706056097HqYv. Password: zToO) prior to publication.
References
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258. https://doi.org/10.1016/s0020-7519(00)00124-7
Elmore SA, Jones JL, Conrad PA, Sharon P, Lindsay DS, Dubey JP (2010) Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol 26:190–196. https://doi.org/10.1016/j.pt.2010.01.009
Dubey JP, Miller NL, Frenkel JK (1970) The Toxoplasma gondii oocyst from cat feces. J Exp Med 132:636–662. https://doi.org/10.1084/jem.132.4.636
Dubey JP (1998) Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 28:1019–1024. https://doi.org/10.1016/s0020-7519(98)00023-x
Sullivan WJ, Victoria J (2012) Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev 36:717–733. https://doi.org/10.1111/j.1574-6976.2011.00305.x
Harker KS, Ueno N, Lodoen MB (2015) Toxoplasma gondii dissemination: a parasite’s journey through the infected host. Parasite Immunol 37:141–149. https://doi.org/10.1111/pim.12163
Dubey JP, Lindsay DS, Speer CA (1998) Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 11:267–299. https://doi.org/10.1128/CMR.11.2.267
Weiss LM, Kim K (2000) The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci 5:D391–D405. https://doi.org/10.2741/weiss
da Silva F, da Fonseca M, Barbosa HS, Uwe G, Lüder CGK (2008) Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol Biosyst 4:824–834. https://doi.org/10.1039/b800520f
Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M (1995) A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol 42:150–157. https://doi.org/10.1111/j.1550-7408.1995.tb01556.x
Kami K (2018) The epigenome, cell cycle, and development in Toxoplasma. Annu Rev Microbiol 72:479–499. https://doi.org/10.1146/annurev-micro-090817-062741
Radke JB, Danielle W, David H, Sherri H, Sullivan WJ, Wilson EH, White MW (2018) Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLoS Pathog 14:e1007035. https://doi.org/10.1371/journal.ppat.1007035
Waldman BS, Dominic S, Wadsworth MH, Saeij JP, Shalek AK, Sebastian L (2020) Identification of a master regulator of differentiation in Toxoplasma. Cell. https://doi.org/10.1016/j.cell.2019.12.013
White MW, Radke JR, Radke JB (2014) Toxoplasma development - turn the switch on or off? Cell Microbiol 16:466–472. https://doi.org/10.1111/cmi.12267
Sinai AP, Suvorova ES (2020) The RESTRICTION checkpoint: a window of opportunity governing developmental transitions in Toxoplasma gondii. Curr Opin Microbiol. https://doi.org/10.1016/j.mib.2020.09.009
Radke JR, Guerini MN, Maria J, White MW (2003) A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol 131:119–127. https://doi.org/10.1016/s0166-6851(03)00198-1
Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW (2001) Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol 115:165–175. https://doi.org/10.1016/s0166-6851(01)00284-5
White MW, Suvorova ES (2018) Apicomplexa cell cycles: something old, borrowed, lost, and new. Trends Parasitol 34:759–771. https://doi.org/10.1016/j.pt.2018.07.006
Francia ME, Boris S (2014) Cell division in apicomplexan parasites. Nat Rev Microbiol 12:125–136. https://doi.org/10.1038/nrmicro3184
Ferguson DJP, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, Gubbels M-J (2008) MORN1 has a conserved role in asexual and sexual development across the apicomplexa. Eukaryot Cell 7:698–711. https://doi.org/10.1128/EC.00021-08
Khelifa AS, Cecilia GS, Lesage KM, Ludovic H, Thomas M, Pierre P, Nicolas B, Helene T, Guillemette M, Emmanuel R et al (2021) TgAP2IX-5 is a key transcriptional regulator of the asexual cell cycle division in Toxoplasma gondii. Nat Commun 12:116. https://doi.org/10.1038/s41467-020-20216-x
Alvarez CA, Suvorova ES (2017) Checkpoints of apicomplexan cell division identified in Toxoplasma gondii. PLoS Pathog 13:e1006483. https://doi.org/10.1371/journal.ppat.1006483
Monavar S, Modarressi Mh, Shojaee S, Mohebali M, Mr E, Mina S, Keshavarz H (2013) Brain tissue cysts in infected mice with RH-strain of Toxoplasma gondii and Evaluation of BAG1 and SAG1 genes expression. Iran J Parasitol 8:40–46
Castro-Elizalde KN, Pedro H-C, Ramírez-Flores CJ, Sirenia G-P, de León G, Carmen T, Mónica M-C, Ricardo M-F (2018) Mycophenolic acid induces differentiation of Toxoplasma gondii RH strain tachyzoites into bradyzoites and formation of cyst-like structure in vitro. Parasitol Res 117:547–563. https://doi.org/10.1007/s00436-017-5738-x
Yuan X, Theisen TC, Suchita R, Abel F, Quake SR, Boothroyd JC (2020) A single-parasite transcriptional atlas of reveals novel control of antigen expression. Elife. https://doi.org/10.7554/eLife.54129
Abdenour S, Stephen D (2016) Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development (Cambridge, England) 143:4301–4311. https://doi.org/10.1242/dev.142075
Stephen D (2015) Linking the cell cycle to cell fate decisions. Trends Cell Biol 25:592–600. https://doi.org/10.1016/j.tcb.2015.07.007
Liu Yi Hu, Huiqing LZ (2013) The cooperative roles of PHO80-like cyclins in regulating the G1/S transition and posterior cytoskeletal morphogenesis in Trypanosoma brucei. Mol Microbiol 90:130–146. https://doi.org/10.1111/mmi.12352
Angel SO, Vanagas L, Ruiz DM, Cristaldi C, Saldarriaga CAM, Sullivan WJ (2020) Emerging therapeutic targets against : update on DNA repair response inhibitors and genotoxic drugs. Front Cell Infect Microbiol 10:289. https://doi.org/10.3389/fcimb.2020.00289
Anne H, Dario A-O, Katharina I, Peter H, Richard L, Nishith G (2013) Optogenetic modulation of an adenylate cyclase in Toxoplasma gondii demonstrates a requirement of the parasite cAMP for host-cell invasion and stage differentiation. J Biol Chem 288:13705–13717. https://doi.org/10.1074/jbc.M113.465583
Kirkman LA, Weiss LM, Kim K (2001) Cyclic nucleotide signaling in Toxoplasma gondii bradyzoite differentiation. Infect Immun 69:148–153. https://doi.org/10.1128/IAI.69.1.148-153.2001
Jia Y, Marq J-B, Bisio H, Jacot D, Mueller C, Yu L, Choudhary J, Brochet M, Soldati-Favre D (2017) Crosstalk between PKA and PKG controls pH-dependent host cell egress of Toxoplasma gondii. EMBO J 36:3250–3267. https://doi.org/10.15252/embj.201796794
Tatsuki S, Fen MY, Tadakimi T, Fumi M, Eaton MS, Rama Y, Han Bing Tu, Vincent KK, Shin-Ichiro K et al (2016) Toxoplasma gondii cyclic AMP-dependent protein kinase subunit 3 is involved in the switch from tachyzoite to bradyzoite development. MBio. https://doi.org/10.1128/mBio.00755-16
Uboldi AD, Mary-Louise W, McRae EA, Stewart RJ, Dagley LF, Luning Y, Katris NJ, Hapuarachchi SV, Coffey MJ, Lehane AM et al (2018) Protein kinase A negatively regulates Ca2+ signalling in Toxoplasma gondii. PLoS Biol 16:e2005642. https://doi.org/10.1371/journal.pbio.2005642
Liliana M-S, Ksenija S, Grilo Ruivo Margarida T, Rita GA, Modrzynska KK, Medina VI, Joana S-D, Rita GA, Ross MC, Pierre C et al (2017) Nutrient sensing modulates malaria parasite virulence. Nature 547:213–216. https://doi.org/10.1038/nature23009
Rita T, Ursula S, Alex B, Ulrike B, Inna C, Peng G, Arnab P, Oliver B (2010) The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8:377–387. https://doi.org/10.1016/j.chom.2010.09.006
Daniel G, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66:789–800. https://doi.org/10.1016/j.molcel.2017.05.032
Shen B, Brown KM, Lee TD, Sibley LD (2014) Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio 5:e01114-e1214. https://doi.org/10.1128/mBio.01114-14
Bang S, Kevin B, Shaojun L, David SL (2017) Development of CRISPR/Cas9 for efficient genome editing in Toxoplasma gondii. Methods Mol Biol (Clifton, NJ). https://doi.org/10.1007/978-1-4939-6472-7_6
Jichao Y, Lihong Z, Huiyan D, Ningbo X, Yanqin Z, Junlong Z, Bang S (2017) ANK1 and DnaK-TPR, two tetratricopeptide repeat-containing proteins primarily expressed in bradyzoites, do not contribute to bradyzoite differentiation. Front Microbiol 8:2210. https://doi.org/10.3389/fmicb.2017.02210
Shu Ye, Ningbo X, Pengfei Z, Jichao Y, Yanqin Z, Bang S, Junlong Z (2019) Micronemal protein 13 contributes to the optimal growth of Toxoplasma gondii under stress conditions. Parasitol Res 118:935–944. https://doi.org/10.1007/s00436-018-06197-3
Congcong L, Xuke Y, Jichao Y, Lun H, Yanqin Z, Junlong Z, Bang S (2021) Role of amylopectin synthesis in and its implication in vaccine development against toxoplasmosis. Open Biol 11:200384. https://doi.org/10.1098/rsob.200384
Robert W, Mathieu G, Croken MM, Ludovic H, David H, Kami K, Stanislas T (2013) The Toxoplasma nuclear factor TgAP2XI-4 controls bradyzoite gene expression and cyst formation. Mol Microbiol 87:641–655. https://doi.org/10.1111/mmi.12121
Buchholz KR, Bowyer PW, Boothroyd JC (2013) Bradyzoite pseudokinase 1 is crucial for efficient oral infectivity of the Toxoplasma gondii tissue cyst. Eukaryot Cell 12:399–410. https://doi.org/10.1128/EC.00343-12
Daehwan K, Ben L, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. https://doi.org/10.1038/nmeth.3317
Mihaela P, Pertea GM, Antonescu CM, Tsung-Cheng C, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33:290–295. https://doi.org/10.1038/nbt.3122
Uboldi AD, McCoy JM, Blume M, Gerlic M, Ferguson DJP, Dagley LF, Beahan CT, Stapleton DI, Gooley PR, Bacic A et al (2015) Regulation of starch stores by a Ca(2+)-dependent protein kinase is essential for viable cyst development in Toxoplasma gondii. Cell Host Microbe 18:670–681. https://doi.org/10.1016/j.chom.2015.11.004
Marcela O, Suparna M, Crosby ME, Alexandru A (2006) Apoptosis assays. Methods Mol Med 129:279–290. https://doi.org/10.1385/1-59745-213-0:279
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023. https://doi.org/10.1038/ncb2329
Carling D, Aguan K, Woods A, Verhoeven AJ, Beri RK, Brennan CH, Sidebottom C, Davison MD, Scott J (1994) Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem 269:11442–11448. https://doi.org/10.1016/S0021-9258(19)78143-5
Pei-Chang S, Ching T, Ban-Hen C, Liu Chen-Wei Yu, Cheng-Ping J-S (2010) Suberoylanilide hydroxamic acid induces apoptosis and sub-G1 arrest of 320 HSR colon cancer cells. J Biomed Sci 17:76. https://doi.org/10.1186/1423-0127-17-76
Lun Z-R, Lai D-H, Wen Y-Z, Zheng L-L, Shen J-L, Yang T-B, Zhou W-L, Qu L-H, Geoff H, Ayala FJ (2015) Cancer in the parasitic protozoans Trypanosoma brucei and Toxoplasma gondii. Proc Natl Acad Sci USA 112:8835–8842. https://doi.org/10.1073/pnas.1502599112
Hee LJ, Hyongjong K, Myungjin K, Yongsung K, Young LS, Karess RE, Sang-Hee L, Minho S, Jin-Man K, Jaeseob K et al (2007) Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447:1017–1020. https://doi.org/10.1038/nature05828
Banko MR, Allen JJ, Schaffer BE, Wilker EW, Peiling T, White JL, Judit V, Beatrice W, Kim SR, Kei S et al (2011) Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol Cell 44:878–892. https://doi.org/10.1016/j.molcel.2011.11.005
Jianlin Lu, Yuanyuan H, Li Z, Wang Ming Xu, Leilei MM, Jianye Z, Guowei F, Zhen D, Xing L et al (2021) AMPKα2 activation by an energy-independent signal ensures chromosomal stability during mitosis. IScience 24:102363. https://doi.org/10.1016/j.isci.2021.102363
Brenman JE (2007) AMPK/LKB1 signaling in epithelial cell polarity and cell division. Cell Cycle (Georgetown, Tex) 6:2755–2759. https://doi.org/10.4161/cc.6.22.4927
Yan Y, Edward ZX, Eric XuH, Karsten M (2018) Structure and physiological regulation of AMPK. Int J Mol Sci. https://doi.org/10.3390/ijms19113534
Grahame HD (2015) Molecular pathways: is AMPK a friend or a foe in cancer? Clin Cancer Res 21:3836–3840. https://doi.org/10.1158/1078-0432.CCR-14-3300
Seth S, Yongji Z, Montserrat S, Jiuli Z, Yuanhong C, Jixin D (2019) Cyclin-dependent kinase 1-mediated AMPK phosphorylation regulates chromosome alignment and mitotic progression. J Cell Sci. https://doi.org/10.1242/jcs.236000
Alejandro V-M, Cristina O-F, Sílvia C, Menendez JA (2011) Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle (Georgetown, Tex) 10:1295–1302. https://doi.org/10.4161/cc.10.8.15342
Acknowledgements
We thank Dr. Zhe Hu from the State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University for support of confocal laser scanning microscopy. We also thank Huiyan Diao, Lihong Zhang and Taifang Zhou for their contributions in the early stage of this project.
Funding
This research was funded by the Guangdong Major Project of Basic and Applied Basic Research (2020B0301030007) and the National Natural Science Foundation of China (No. 31822054).
Author information
Authors and Affiliations
Contributions
JY and BS designed the study; JY performed the experiments; PW, CP, AL, XY and CL contributed significantly to phospho-proteomic experiments; ZN and XY contributed significantly to Western blot assays; CL, AL, XY and XL contributed significantly to animal experiments; NX, JC and ML helped with data analyses; XY and YL were involved in discussions and helped with the designs of the experiments; JY and BS performed the data analyses and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
All animal experiments were approved by The Scientific Ethic Committee of Huazhong Agricultural University (permit #: HZAUMO-2020–0071).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Yang, X., Liu, A. et al. The beta subunit of AMP-activated protein kinase is critical for cell cycle progression and parasite development in Toxoplasma gondii. Cell. Mol. Life Sci. 79, 532 (2022). https://doi.org/10.1007/s00018-022-04556-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04556-z