Abstract
Background
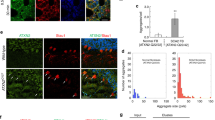
Poly-GA, a dipeptide repeat protein unconventionally translated from GGGGCC (G4C2) repeat expansions in C9orf72, is abundant in C9orf72-related amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (C9orf72-ALS/FTD). Although the poly-GA aggregates have been identified in C9orf72-ALS/FTD neurons, the effects on UPS (ubiquitin–proteasome system) and autophagy and their exact molecular mechanisms have not been fully elucidated.
Results
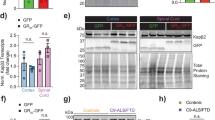
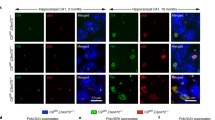
Herein, our in vivo experiments indicate that the mice expressing ploy-GA with 150 repeats instead of 30 repeats exhibit significant aggregates in cells. Mice expressing 150 repeats ploy-GA shows behavioral deficits and activates autophagy in the brain. In vitro findings suggest that the poly-GA aggregates influence proteasomal by directly binding proteasome subunit PSMD2. Subsequently, the poly-GA aggregates activate phosphorylation and ubiquitination of p62 to recruit autophagosomes. Ultimately, the poly-GA aggregates lead to compensatory activation of autophagy. In vivo studies further reveal that rapamycin (autophagy activator) treatment significantly improves the degenerative symptoms and alleviates neuronal injury in mice expressing 150 repeats poly-GA. Meanwhile, rapamycin administration to mice expressing 150 repeats poly-GA reduces neuroinflammation and aggregates in the brain.
Conclusion
In summary, we elucidate the relationship between poly-GA in the proteasome and autophagy: when poly-GA forms complexes with the proteasome, it recruits autophagosomes and affects proteasome function. Our study provides support for further promoting the comprehension of the pathogenesis of C9orf72, which may bring a hint for the exploration of rapamycin for the treatment of ALS/FTD.








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. Further data supporting the findings of this study are available from the corresponding authors on request.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- FTD:
-
Frontotemporal dementia
- C9orf72:
-
Chromosome 9 open reading frame 72
- poly-GA:
-
Poly-Glycine Alanine
- GFAP:
-
Glial fibrillary acidic protein
- Iba1:
-
Ionized calcium-binding adapter molecule 1
- UPS:
-
Ubiquitin − proteasome system
- DPRs:
-
Dipeptide repeat proteins
- PolyQ:
-
Polyglutamine
- HD:
-
Huntington’s disease
- AD:
-
Alzheimer’s disease
- LIR:
-
LC3-interacting region
References
DeJesus-Hernandez M et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72(2):245–256
Renton AE et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72(2):257–268
Haeusler AR et al (2014) C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 507(7491):195–200
Mori K et al (2013) Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol 126(6):881–893
Mori K et al (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339(6125):1335–1338
Mackenzie IR et al (2013) Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol 126(6):859–879
Mackenzie IR et al (2015) Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol 130(6):845–861
Mizielinska S et al (2014) C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science 345(6201):1192–1194
Tao Z et al (2015) Nucleolar stress and impaired stress granule formation contribute to C9orf72 RAN translation-induced cytotoxicity. Hum Mol Genet 24(9):2426–2441
Zhang YJ et al (2014) Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol 128(4):505–524
Zhang YJ et al (2016) C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins. Nat Neurosci 19(5):668–677
Schludi MH et al (2017) Spinal poly-GA inclusions in a C9orf72 mouse model trigger motor deficits and inflammation without neuron loss. Acta Neuropathol 134(2):241–254
Gijselinck I et al (2016) The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol Psychiatry 21(8):1112–1124
Ying Z et al (2009) Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet 18(22):4268–4281
Sun J et al (2018) Inhibition of p70 S6 kinase activity by A77 1726 induces autophagy and enhances the degradation of superoxide dismutase 1 (SOD1) protein aggregates. Cell Death Dis. https://doi.org/10.1038/s41419-018-0441-0
Samant RS et al (2018) Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control. Nature 563(7731):407–411
Zhao JH et al (2015) mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci USA 112(52):15790–15797
Finley D (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78:477–513
Collins GA, Goldberg AL (2017) The logic of the 26S proteasome. Cell 169(5):792–806
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873
Pankiv S et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282(33):24131–24145
Waite KA et al (2016) Starvation induces proteasome autophagy with different pathways for core and regulatory particles. J Biol Chem 291(7):3239–3253
Zhou Q et al (2017) Antibodies inhibit transmission and aggregation of C9orf72 poly-GA dipeptide repeat proteins. EMBO Mol Med 9(5):687–702
Edbauer D (2016) Poly-GA initiates the disease cascade in C9orf72 FTLD/ALS. J Neurochem 138:252–252
Deverman BE et al (2016) Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34(2):204–209
Chan KY et al (2017) Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20(8):1172–1179
McCauley ME, Baloh RH (2019) Inflammation in ALS/FTD pathogenesis. Acta Neuropathol 137(5):715–730
Ma XY et al (2022) CCT2 is an aggrephagy receptor for clearance of solid protein aggregates. Cell 185(8):1325–1345
Chai Y et al (1999) Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet 8(4):673–682
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292(5521):1552–1555
Neefjes J, Dantuma NP (2004) Fluorescent probes for proteolysis: Tools for drug discovery. Nat Rev Drug Discov 3(1):58–69
Juszkiewicz S, Hegde RS (2018) Quality control of orphaned proteins. Mol Cell 71(3):443–457
Bett JS (2016) Proteostasis regulation by the ubiquitin system. Proteostasis 60(2):143–151
Cohen-Kaplan V et al (2016) p62-and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc Natl Acad Sci USA 113(47):E7490–E7499
Kawaguchi Y et al (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115(6):727–738
McNaught KSP et al (2002) Aggresome-related biogenesis of Lewy bodies. Eur J Neurosci 16(11):2136–2148
Boughton AJ et al (2021) Polyubiquitin and ubiquitin-like signals share common recognition sites on proteasomal subunit Rpn1. J Biol Chem 296:100450
Kliosnky D (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) (vol 12, pg 1, 2015). Autophagy 12(2):443
Li X et al (2021) Annexin-A1 SUMOylation regulates microglial polarization after cerebral ischemia by modulating IKK alpha stability via selective autophagy. Sci Adv. https://doi.org/10.1126/sciadv.abc5539
Guo H et al (2020) Nuclear miR-30b-5p suppresses TFEB-mediated lysosomal biogenesis and autophagy. Cell Death Differ 28(1):320–336
Tanji K et al (2014) Phosphorylation of serine 349 of p62 in Alzheimer’s disease brain. Acta Neuropathologica Commun. https://doi.org/10.1186/2051-5960-2-50
Matsumoto G et al (2011) Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell 44(2):279–289
Pilli M et al (2012) TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37(2):223–234
Ichimura Y et al (2013) Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 51(5):618–631
Shi Y et al (2016) Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science. https://doi.org/10.1126/science.aad9421
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47(3):e147
Cohen-Kaplan V et al (2016) p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc Natl Acad Sci USA 113(47):E7490–E7499
Kaiho-Soma A et al (2021) TRIP12 promotes small-molecule-induced degradation through K29/K48-branched ubiquitin chains. Mol Cell 81(7):1411–1424 (e7)
Banerji V, Gibson SB (2012) Targeting metabolism and autophagy in the context of haematologic malignancies. Int J Cell Biol 2012:595976
Park SH et al (2013) PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell 154(1):134–145
Kristiansen M et al (2007) Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell 26(2):175–188
Guo Q et al (2018) In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell 172(4):696–705
Cuervo AM et al (1995) Degradation of proteasomes by lysosomes in rat-liver. Eur J Biochem 227(3):792–800
Marshall RS et al (2015) Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/ubiquitin receptor RPN10 in Arabidopsis. Mol Cell 58(6):1053–1066
Lu KF, Psakhye I, Jentsch S (2014) Autophagic clearance of PolyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 158(3):549–563
Kageyama S et al (2014) Proteasome dysfunction activates autophagy and the Keap1-Nrf2 pathway. J Biol Chem 289(36):24944–24955
Nguyen L et al (2020) Antibody therapy targeting RAN proteins rescues C9 ALS/FTD phenotypes in C9orf72 mouse model. Neuron 105(4):645–662
Zhang K et al (2021) UBQLN2-HSP70 axis reduces poly-Gly-Ala aggregates and alleviates behavioral defects in the C9ORF72 animal model. Neuron 109(12):1949–1962 (e6)
Zhang XJ et al (2011) Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 7(4):412–425
Caccamo A et al (2009) Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability. J Biol Chem 284(40):27416–27424
Wang IF et al (2012) Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci USA 109(37):15024–15029
Mandrioli J et al (2018) Rapamycin treatment for amyotrophic lateral sclerosis Protocol for a phase II randomized, double-blind, placebo-controlled, multicenter, clinical trial (RAP-ALS trial). Medicine 97(24):e11119
Liu Y, Wang J (2019) C9orf72-dependent lysosomal functions regulate epigenetic control of autophagy and lipid metabolism. Autophagy 15(5):913–914
Ho WY et al (2019) The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15(5):827–842
Yang M et al (2016) A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv. https://doi.org/10.1126/sciadv.1601167
Zhou L et al (2014) p62/sequestosome 1 regulates aggresome formation of pathogenic ataxin-3 with expanded polyglutamine. Int J Mol Sci 15(9):14997–15010
Morrow CS et al (2020) Vimentin coordinates protein turnover at the aggresome during neural stem cell quiescence exit. Cell Stem Cell 26(4):558–568 (e9)
Acknowledgements
We would like to thank Dr. Ruimin Huang (Shanghai Institute of Materia Medica) for his critical comments and suggestions on our manuscript; Thank Dr. Kai Fu (Xiangya Hospital Center South University) for his advice and critical reading of this manuscript. Thank Dr. Zhaobing Gao (Shanghai Institute of Materia Medica) for helpful comments.
Funding
This work was supported by the Foundation of Shanghai Science and Technology Committee (No. 21ZR1475100, 21DZ2291100), the Fundamental Research Funds for the State Key Laboratory of Drug Research (SIMM2103ZZ-01), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-D-202210).
Author information
Authors and Affiliations
Contributions
All the authors contributed significantly to the study. MP, ZT: conceptualization. MP, YT, HG, LY, YZ, ZH: methodology. MP: software. MP, ZT: writing—original draft. GW, ZT, JR: writing—reviewing and editing. ZT, JC, XQ, JR: supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethics approval and consent to participate
No human data or tissue were involved. All animal procedures received approval from the Animal Care and Use Committee of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Shanghai, China).
Consent for publication
We have obtained consent to publish this paper from all the participants of this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pu, M., Tai, Y., Yuan, L. et al. The contribution of proteasomal impairment to autophagy activation by C9orf72 poly-GA aggregates. Cell. Mol. Life Sci. 79, 501 (2022). https://doi.org/10.1007/s00018-022-04518-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04518-5