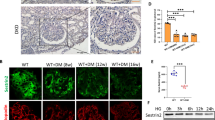
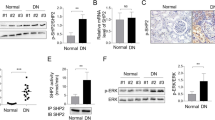
Abstract
Diabetic nephropathy (DN) is a significant complication of diabetes and the leading cause of end-stage renal disease. Hyperglycemia-induced dysfunction of the glomerular podocytes is a major contributor to the deterioration of renal function in DN. Previously, we demonstrated that podocyte-specific disruption of the Src homology phosphatase 2 (Shp2) ameliorated lipopolysaccharide-induced renal injury. This study aims to evaluate the contribution of Shp2 to podocyte function under hyperglycemia and explore the molecular underpinnings. We report elevated Shp2 in the E11 podocyte cell line under high glucose and the kidney under streptozotocin- and high-fat diet-induced hyperglycemia. Consistently, Shp2 disruption in podocytes was associated with partial renoprotective effects under hyperglycemia, as evidenced by the preserved renal function. At the molecular level, Shp2 deficiency was associated with altered renal insulin signaling and diminished hyperglycemia-induced renal endoplasmic reticulum stress, inflammation, and fibrosis. Additionally, Shp2 knockdown in E11 podocytes mimicked the in vivo deficiency of this phosphatase and ameliorated the deleterious impact of high glucose, whereas Shp2 reconstitution reversed these effects. Moreover, Shp2 deficiency attenuated high glucose-induced E11 podocyte migration. Further, we identified the protein tyrosine kinase FYN as a putative mediator of Shp2 signaling in podocytes under high glucose. Collectively, these findings suggest that Shp2 inactivation may afford protection to podocytes under hyperglycemia and highlight this phosphatase as a potential target to ameliorate glomerular dysfunction in DN.







Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Kramer A, Pippias M, Noordzij M, Stel VS, Andrusev AM, Aparicio-Madre MI, Arribas Monzon FE, Asberg A, Barbullushi M, Beltran P, Bonthuis M, Caskey FJ, Castro de la Nuez P, Cernevskis H, De Meester J, Finne P, Golan E, Heaf JG, Hemmelder MH, Ioannou K, Kantaria N, Komissarov K, Korejwo G, Kramar R, Lassalle M, Lopot F, Macario F, Mackinnon B, Palsson R, Pechter U, Pinera VC, Santiuste de Pablos C, Segarra-Medrano A, Seyahi N, Slon Roblero MF, Stojceva-Taneva O, Vazelov E, Winzeler R, Ziginskiene E, Massy Z, Jager KJ (2019) The European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2016: a summary. Clin Kidney J 12(5):702–720
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895
Jitraknatee J, Ruengorn C, Nochaiwong S (2020) Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: a cross-sectional study in primary care practice. Sci Rep 10(1):6205
Kainz A, Hronsky M, Stel VS, Jager KJ, Geroldinger A, Dunkler D, Heinze G, Tripepi G, Oberbauer R (2015) Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transpl 30(4):iv113–iv118
McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM (2019) Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol 30(1):127–135
Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cao J, Chen JL, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert H, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar-Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez-Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V (2017) US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 69(3 Suppl 1):A7–A8
Lin JS, Susztak K (2016) Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep 16(5):45
Greka A, Mundel P (2012) Cell biology and pathology of podocytes. Annu Rev Physiol 74:299–323
Gil CL, Hooker E, Larrivee B (2021) Diabetic kidney disease endothelial damage, and podocyte-endothelial crosstalk. Kidney Med 3(1):105–115
Wolf G, Chen S, Ziyadeh FN (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54(6):1626–1634
Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC (2005) Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16(10):2941–2952
Kravets I, Mallipattu SK (2020) The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. J Endocr Soc 4(4):bvaa029
Lu CC, Wang GH, Lu J, Chen PP, Zhang Y, Hu ZB, Ma KL (2019) Role of Podocyte Injury in Glomerulosclerosis. Adv Exp Med Biol 1165:195–232
Lay AC, Coward RJM (2018) The evolving importance of insulin signaling in podocyte health and disease. Front Endocrinol (Lausanne) 9:693
Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavare JM, Mathieson PW, Saleem MA (2005) The human glomerular podocyte is a novel target for insulin action. Diabetes 54(11):3095–3102
Rask-Madsen C, King GL (2010) Diabetes: podocytes lose their footing. Nature 468(7320):42–44
Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ (2010) Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12(4):329–340
Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, Ranjan S, Wolter J, Kohli S, Shahzad K, Heidel F, Krueger M, Schwenger V, Moeller MJ, Kalinski T, Reiser J, Chavakis T, Isermann B (2015) Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun 6:6496
Lay AC, Hurcombe JA, Betin VMS, Barrington F, Rollason R, Ni L, Gillam L, Pearson GME, Ostergaard MV, Hamidi H, Lennon R, Welsh GI, Coward RJM (2017) Prolonged exposure of mouse and human podocytes to insulin induces insulin resistance through lysosomal and proteasomal degradation of the insulin receptor. Diabetologia 60(11):2299–2311
Kim EY, Anderson M, Dryer SE (2012) Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 302(3):F298-307
Xia S, Liu Y, Li X, Thilo F, Tepel M (2016) Insulin increases expression of TRPC6 channels in podocytes by a calcineurin-dependent pathway. Cell Physiol Biochem 38(2):659–669
Geraldes P (2018) Protein phosphatases and podocyte function. Curr Opin Nephrol Hypertens 27(1):49–55
Aoudjit L, Jiang R, Lee TH, New LA, Jones N, Takano T (2011) Podocyte protein, nephrin, is a substrate of protein tyrosine phosphatase 1B. J Signal Transduct 2011:376543
Ito Y, Hsu MF, Bettaieb A, Koike S, Mello A, Calvo-Rubio M, Villalba JM, Haj FG (2017) Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metabolism 76:56–69
Denhez B, Lizotte F, Guimond MO, Jones N, Takano T, Geraldes P (2015) Increased SHP-1 protein expression by high glucose levels reduces nephrin phosphorylation in podocytes. J Biol Chem 290(1):350–358
Drapeau N, Lizotte F, Denhez B, Guay A, Kennedy CR, Geraldes P (2013) Expression of SHP-1 induced by hyperglycemia prevents insulin actions in podocytes. Am J Physiol Endocrinol Metab 304(11):E1188–E1198
Lizotte F, Denhez B, Guay A, Gevry N, Cote AM, Geraldes P (2016) Persistent insulin resistance in podocytes caused by epigenetic changes of SHP-1 in diabetes. Diabetes 65(12):3705–3717
Sugimoto S, Lechleider RJ, Shoelson SE, Neel BG, Walsh CT (1993) Expression, purification, and characterization of SH2-containing protein tyrosine phosphatase, SH-PTP2. J Biol Chem 268(30):22771–22776
Feng GS (2007) Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res 17(1):37–41
Chan G, Kalaitzidis D, Neel BG (2008) The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev 27(2):179–192
Maile LA, Clemmons DR (2002) Regulation of insulin-like growth factor I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. J Biol Chem 277(11):8955–8960
Ling Y, Maile LA, Clemmons DR (2003) Tyrosine phosphorylation of the beta3-subunit of the alphaVbeta3 integrin is required for embrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol 17(9):1824–1833
Dixit M, Zhuang D, Ceacareanu B, Hassid A (2003) Treatment with insulin uncovers the motogenic capacity of nitric oxide in aortic smooth muscle cells: dependence on Gab1 and Gab1-SHP2 association. Circ Res 93(10):e113–e123
Mussig K, Staiger H, Fiedler H, Moeschel K, Beck A, Kellerer M, Haring HU (2005) Shp2 is required for protein kinase C-dependent phosphorylation of serine 307 in insulin receptor substrate-1. J Biol Chem 280(38):32693–32699
Hall C, Yu H, Choi E (2020) Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp Mol Med 52(6):911–920
Matsuo K, Delibegovic M, Matsuo I, Nagata N, Liu S, Bettaieb A, Xi Y, Araki K, Yang W, Kahn BB, Neel BG, Haj FG (2010) Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J Biol Chem 285(51):39750–39758
Princen F, Bard E, Sheikh F, Zhang SS, Wang J, Zago WM, Wu D, Trelles RD, Bailly-Maitre B, Kahn CR, Chen Y, Reed JC, Tong GG, Mercola M, Chen J, Feng GS (2009) Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol Cell Biol 29(2):378–388
Choi E, Kikuchi S, Gao H, Brodzik K, Nassour I, Yopp A, Singal AG, Zhu H, Yu H (2019) Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat Commun 10(1):1473
Verma R, Venkatareddy M, Kalinowski A, Patel SR, Salant DJ, Garg P (2015) Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury. Mol Cell Biol 36(4):596–614
Hsu MF, Bettaieb A, Ito Y, Graham J, Havel PJ, Haj FG (2017) Protein tyrosine phosphatase Shp2 deficiency in podocytes attenuates lipopolysaccharide-induced proteinuria. Sci Rep 7(1):461
Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, Neel BG (2004) Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell 13(3):341–355
Agazie YM, Hayman MJ (2003) Development of an efficient “substrate-trapping” mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J Biol Chem 278(16):13952–13958
Cybulsky AV (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13(11):681–696
Lv Z, Hu M, Ren X, Fan M, Zhen J, Chen L, Lin J, Ding N, Wang Q, Wang R (2016) Fyn mediates high glucose-induced actin cytoskeleton reorganization of podocytes via promoting ROCK activation in vitro. J Diabetes Res 2016:5671803
Saito YD, Jensen AR, Salgia R, Posadas EM (2010) Fyn: a novel molecular target in cancer. Cancer 116(7):1629–1637
Kumar A, Jaggi AS, Singh N (2015) Pharmacology of Src family kinases and therapeutic implications of their modulators. Fundam Clin Pharmacol 29(2):115–130
Roskoski R Jr (2005) Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 331(1):1–14
Fang X, Lang Y, Wang Y, Mo W, Wei H, Xie J, Yu M (2012) Shp2 activates Fyn and Ras to regulate RBL-2H3 mast cell activation following FcepsilonRI aggregation. PLoS ONE 7(7):e40566
Cheng Y, Wang D, Wang F, Liu J, Huang B, Baker MA, Yin J, Wu R, Liu X, Regner KR, Usa K, Liu Y, Zhang C, Dong L, Geurts AM, Wang N, Miller SS, He Y, Liang M (2020) Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J Am Soc Nephrol 31(7):1539–1554
Kumagai T, Baldwin C, Aoudjit L, Nezvitsky L, Robins R, Jiang R, Takano T (2014) Protein tyrosine phosphatase 1B inhibition protects against podocyte injury and proteinuria. Am J Pathol 184(8):2211–2224
Wang J, Mizui M, Zeng LF, Bronson R, Finnell M, Terhorst C, Kyttaris VC, Tsokos GC, Zhang ZY, Kontaridis MI (2016) Inhibition of SHP2 ameliorates the pathogenesis of systemic lupus erythematosus. J Clin Invest 126(6):2077–2092
Jiang J, Hu B, Chung CS, Chen Y, Zhang Y, Tindal EW, Li J, Ayala A (2020) SHP2 inhibitor PHPS1 ameliorates acute kidney injury by Erk1/2-STAT3 signaling in a combined murine hemorrhage followed by septic challenge model. Mol Med 26(1):89
do Carmo JM, da Silva AA, Ebaady SE, Sessums PO, Abraham RS, Elmquist JK, Lowell BB, Hall JE (2014) Shp2 signaling in POMC neurons is important for leptin’s actions on blood pressure, energy balance, and glucose regulation. Am J Physiol Regul Integr Comp Physiol 307(12):R1438–R1447
Wang Z, do Carmo JM, da Silva AA, Fu Y, Hall JE (2020) Mechanisms of synergistic interactions of diabetes and hypertension in chronic kidney disease: role of mitochondrial dysfunction and ER stress. Curr Hypertens Rep 22(2):15
Nagata N, Matsuo K, Bettaieb A, Bakke J, Matsuo I, Graham J, Xi Y, Liu S, Tomilov A, Tomilova N, Gray S, Jung DY, Ramsey JJ, Kim JK, Cortopassi G, Havel PJ, Haj FG (2012) Hepatic Src homology phosphatase 2 regulates energy balance in mice. Endocrinology 153(7):3158–3169
Wang HC, Zhou Y, Huang SK (2017) SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Arch Toxicol 91(4):1739–1748
Teng JF, Wang K, Jia ZM, Guo YJ, Guan YW, Li ZH, Ai X (2018) Lentivirus-mediated silencing of Src homology 2 domain-containing protein tyrosine phosphatase 2 inhibits release of inflammatory cytokines and apoptosis in renal tubular epithelial cells via inhibition of the TLR4/NF-kB pathway in renal ischemia-reperfusion injury. Kidney Blood Press Res 43(4):1084–1103
Zehender A, Huang J, Gyorfi AH, Matei AE, Trinh-Minh T, Xu X, Li YN, Chen CW, Lin J, Dees C, Beyer C, Gelse K, Zhang ZY, Bergmann C, Ramming A, Birchmeier W, Distler O, Schett G, Distler JHW (2018) The tyrosine phosphatase SHP2 controls TGFbeta-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat Commun 9(1):3259
Leung JC, Lai KN, Tang SCW (2014) Crosstalk between podocytes and tubular epithelial cells. Podocytopathy 183:54–63
Fu J, Shinjo T, Li Q, St-Louis R, Park K, Yu MG, Yokomizo H, Simao F, Huang Q, Wu IH, King GL (2022) Regeneration of glomerular metabolism and function by podocyte pyruvate kinase M2 in diabetic nephropathy. JCI Insight 7(5):e155260
Canaud G, Bienaime F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F (2013) AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19(10):1288–1296
Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A (2008) Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int 73(12):1385–1393
Feliers D, Kasinath BS (2011) Erk in kidney diseases. J Signal Transduct 2011:768512
Isshiki K, Haneda M, Koya D, Maeda S, Sugimoto T, Kikkawa R (2000) Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes 49(6):1022–1032
Liu S, Ding J, Fan Q, Zhang H (2010) The activation of extracellular signal-regulated kinase is responsible for podocyte injury. Mol Biol Rep 37(5):2477–2484
Xu E, Schwab M, Marette A (2014) Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord 15(1):79–97
Villarreal R, Mitrofanova A, Maiguel D, Morales X, Jeon J, Grahammer F, Leibiger IB, Guzman J, Fachado A, Yoo TH, Busher Katin A, Gellermann J, Merscher S, Burke GW, Berggren PO, Oh J, Huber TB, Fornoni A (2016) Nephrin contributes to insulin secretion and affects mammalian target of rapamycin signaling independently of insulin receptor. J Am Soc Nephrol 27(4):1029–1041
Liu Q, Qu J, Zhao M, Xu Q, Sun Y (2020) Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res 152:104595
Yuan X, Bu H, Zhou J, Yang CY, Zhang H (2020) Recent advances of SHP2 inhibitors in cancer therapy: current development and clinical application. J Med Chem 63(20):11368–11396
Acknowledgements
Research in the Haj laboratory was funded by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK095359 and R01DK090492, the National Institute of Environmental Health Sciences grant P42ES04699, and NIFA grant CA-D*-NTR-7836H. Dr. Haj is a Co-Leader of the Endocrinology and Metabolism Core of UC Davis Mouse Metabolic Phenotyping Center, which is funded by U24DK092993. Dr. Hsu was supported by National Institute on Alcohol Abuse and Alcoholism grant R21AA027633. Dr. Afkarian was supported by the grant R01DK104706 from the National Institute of Diabetes and Digestive and Kidney Diseases. The Light Microscopy Imaging Facility (UC Davis) is supported by National Institutes of Health grant 1S10RR019266.
Funding
Research in the Haj laboratory was funded by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK095359 and R01DK090492, the National Institute of Environmental Health Sciences grant P42ES04699, and NIFA grant CA-D*-NTR-7836H. Dr. Haj is a Co-Leader of the Endocrinology and Metabolism Core of UC Davis Mouse Metabolic Phenotyping Center, which is funded by U24DK092993. Dr. Hsu was supported by National Institute on Alcohol Abuse and Alcoholism grant R21AA027633. Dr. Afkarian was supported by the grant R01DK104706 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Contributions
MH, YI, and FH contributed to the study design and interpretation of the data. MH and YI conducted the experiments and analyzed the data. MH wrote the first draft of the manuscript. MH, YI, MA, and FH reviewed and edited the manuscript. All authors read and approved the submission of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors disclose no conflicts associated with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsu, MF., Ito, Y., Afkarian, M. et al. Deficiency of the Src homology phosphatase 2 in podocytes is associated with renoprotective effects in mice under hyperglycemia. Cell. Mol. Life Sci. 79, 516 (2022). https://doi.org/10.1007/s00018-022-04517-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04517-6