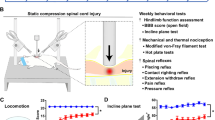
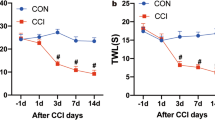
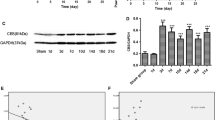
Abstract
Intractable neuropathic pain following spinal cord injury (NP-SCI) reduces a patient’s quality of life. Excessive release of ATP into the extracellular space evokes neuroinflammation via purinergic receptor. Neuroinflammation plays an important role in the initiation and maintenance of NP. However, little is known about whether or not extracellular ATP cause NP-SCI. We found in the present study that excess of intracellular ATP at the lesion site evokes at-level NP-SCI. No significant differences in the body weight, locomotor function, or motor behaviors were found in groups that were negative and positive for at-level allodynia. The intracellular ATP level at the lesion site was significantly higher in the allodynia-positive mice than in the allodynia-negative mice. A metabolome analysis revealed that there were no significant differences in the ATP production or degradation between allodynia-negative and allodynia-positive mice. Dorsal horn neurons in allodynia mice were found to be inactivated in the resting state, suggesting that decreased ATP consumption due to neural inactivity leads to a build-up of intracellular ATP. In contrast to the findings in the resting state, mechanical stimulation increased the neural activity of dorsal horn and extracellular ATP release at lesion site. The forced production of intracellular ATP at the lesion site in non-allodynia mice induced allodynia. The inhibition of P2X4 receptors in allodynia mice reduced allodynia. These results suggest that an excess buildup of intracellular ATP in the resting state causes at-level NP-SCI as a result of the extracellular release of ATP with mechanical stimulation.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKT:
-
Protein kinase B
- BDNF:
-
Brain-derived neurotrophic factor
- FRET:
-
Förster resonance energy transfer
- GABA:
-
Gamma-amino-butyric-acid
- JNK:
-
C-Jun N-terminal kinase
- KCC2:
-
K+–Cl− cotransporter 2
- MAPK:
-
Mitogen-activated protein kinase
- MRI:
-
Magnetic resonance imaging
- NLRP:
-
NOD-, LRR- and pyrin domain-containing protein
- NMDA:
-
N-methyl-D-aspartate
- NP:
-
Neuropathic pain
- P2X4:
-
P2X prinoreceptor 4
- SCI:
-
Spinal cord injury
References
von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73(4):638–652. https://doi.org/10.1016/j.neuron.2012.02.008
Ravenscroft A, Ahmed YS, Burnside IG (2000) Chronic pain after SCI A patient survey. Spinal cord 38(10):611–614. https://doi.org/10.1038/sj.sc.3101073
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ (2003) A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103(3):249–257. https://doi.org/10.1016/S0304-3959(02)00452-9
Rintala DH, Loubser PG, Castro J, Hart KA, Fuhrer MJ (1998) Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Arch Phys Med Rehabil 79(6):604–614. https://doi.org/10.1016/s0003-9993(98)90032-6
Störmer S, Gerner HJ, Grüninger W, Metzmacher K, Föllinger S, Wienke C, Aldinger W, Walker N, Zimmermann M, Paeslack V (1997) Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal cord 35(7):446–455. https://doi.org/10.1038/sj.sc.3100411
Siddall PJ, Middleton JW (2015) Spinal cord injury-induced pain: mechanisms and treatments. Pain Manag 5(6):493–507. https://doi.org/10.2217/pmt.15.47
McMahon SB, Malcangio M (2009) Current challenges in glia-pain biology. Neuron 64(1):46–54. https://doi.org/10.1016/j.neuron.2009.09.033
Nakagawa T, Kaneko S (2010) Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci 114(4):347–353. https://doi.org/10.1254/jphs.10r04cp
Zhao P, Waxman SG, Hains BC (2007) Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27(9):2357–2368. https://doi.org/10.1523/JNEUROSCI.0138-07.2007
Theriault E, Frankenstein UN, Hertzberg EL, Nagy JI (1997) Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J Comp Neurol 382(2):199–214
Huang C, Han X, Li X, Lam E, Peng W, Lou N, Torres A, Yang M, Garre JM, Tian GF, Bennett MV, Nedergaard M, Takano T (2012) Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci 32(10):3333–3338. https://doi.org/10.1523/JNEUROSCI.1216-11.2012
Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M (2009) Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA 106(30):12489–12493. https://doi.org/10.1073/pnas.0902531106
Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M (2012) Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60(11):1660–1670. https://doi.org/10.1002/glia.22384
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6):752–758. https://doi.org/10.1038/nn1472
Zhang ZJ, Jiang BC, Gao YJ (2017) Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 74(18):3275–3291. https://doi.org/10.1007/s00018-017-2513-1
Pfyffer D, Wyss PO, Huber E, Curt A, Henning A, Freund P (2020) Metabolites of neuroinflammation relate to neuropathic pain after spinal cord injury. Neurology 95(7):e805–e814. https://doi.org/10.1212/WNL.0000000000010003
Trang T, Salter MW (2012) P2X4 purinoceptor signaling in chronic pain. Purinergic Signal 8(3):621–628. https://doi.org/10.1007/s11302-012-9306-7
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424(6950):778–783. https://doi.org/10.1038/nature01786
Schwab JM, Guo L, Schluesener HJ (2005) Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol 163(1–2):185–189. https://doi.org/10.1016/j.jneuroim.2005.02.016
de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S (2012) P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci 32(9):3058–3066. https://doi.org/10.1523/JNEUROSCI.4930-11.2012
Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE (2008) Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol 213(2):257–267. https://doi.org/10.1016/j.expneurol.2008.05.025
Hains BC, Waxman SG (2006) Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26(16):4308–4317. https://doi.org/10.1523/JNEUROSCI.0003-06.2006
Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19(3):138–152. https://doi.org/10.1038/nrn.2018.2
Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K (2013) P2X4 receptors and neuropathic pain. Front Cell Neurosci 7:191. https://doi.org/10.3389/fncel.2013.00191
Nakano M, Imamura H, Nagai T, Noji H (2011) Ca2+ regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol 6(7):709–715. https://doi.org/10.1021/cb100313n
Yamamoto M, Kim M, Imai H, Itakura Y, Ohtsuki G (2019) Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Rep 28(11):2923-2938.e8. https://doi.org/10.1016/j.celrep.2019.07.078
Long T, He W, Pan Q, Zhang S, Zhang Y, Liu C, Liu Q, Qin G, Chen L, Zhou J (2018) Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J Neuroinflammation 15(1):245. https://doi.org/10.1186/s12974-018-1285-3
Minett MS, Eijkelkamp N, Wood JN (2014) Significant determinants of mouse pain behaviour. PLoS ONE 9(8):e104458. https://doi.org/10.1371/journal.pone.0104458
Lei BH, Chen JH, Yin HS (2014) Repeated amphetamine treatment alters spinal magnetic resonance signals and pain sensitivity in mice. Neurosci Lett 583:70–75. https://doi.org/10.1016/j.neulet.2014.09.031
Kikuta S, Nakamura Y, Yamamura Y, Tamura A, Homma N, Yanagawa Y, Tamura H, Kasahara J, Osanai M (2015) Quantitative activation-induced manganese-enhanced MRI reveals severity of Parkinson’s disease in mice. Sci Rep 5:12800. https://doi.org/10.1038/srep12800
Furue H, Narikawa K, Kumamoto E, Yoshimura M (1999) Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J Physiol 521(Pt 2):529–535. https://doi.org/10.1111/j.1469-7793.1999.00529.x
Furue H, Sonohata M, Yoshimura M (2003) Nihon seirigaku zasshi. J Physiol Soc Jpn 65(10):315–321
Sugiyama D, Hur SW, Pickering AE, Kase D, Kim SJ, Kawamata M, Imoto K, Furue H (2012) In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J Physiol 590(10):2225–2231. https://doi.org/10.1113/jphysiol.2011.226407
Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, Imoto K, Furue H (2014) Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain 155(3):617–628. https://doi.org/10.1016/j.pain.2013.12.018
Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT (2005) Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 280(6):4761–4771. https://doi.org/10.1074/jbc.M409650200
Ying R, Liang HL, Whelan HT, Eells JT, Wong-Riley MT (2008) Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Res 1243:167–173. https://doi.org/10.1016/j.brainres.2008.09.057
Yu Z, Liu N, Zhao J, Li Y, McCarthy TJ, Tedford CE, Lo EH, Wang X (2015) Near infrared radiation rescues mitochondrial dysfunction in cortical neurons after oxygen-glucose deprivation. Metab Brain Dis 30(2):491–496. https://doi.org/10.1007/s11011-014-9515-6
Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K (2002) Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 323(3):207–210. https://doi.org/10.1016/s0304-3940(02)00159-3
Ohnishi Y, Yamamoto M, Sugiura Y, Setoyama D, Kishima H (2021) Rostro-caudal different energy metabolism leading to differences in degeneration in spinal cord injury. Brain communications 3(2):fcab058. https://doi.org/10.1093/braincomms/fcab058
Brambilla D, Chapman D, Greene R (2005) Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron 46(2):275–283. https://doi.org/10.1016/j.neuron.2005.03.016
Manzoni OJ, Manabe T, Nicoll RA (1994) Release of adenosine by activation of NMDA receptors in the hippocampus. Science (New York, NY) 265(5181):2098–2101. https://doi.org/10.1126/science.7916485
Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M (2012) Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA 109(16):6265–6270. https://doi.org/10.1073/pnas.1120997109
Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT, Graves SM, Uweru JO, Ledderose C, Kutlu MG, Wheeler MA, Kahan A, Ishikawa M, Wang YC, Loh YE, Jiang JX, Surmeier DJ, Robson SC, Schaefer A (2020) Negative feedback control of neuronal activity by microglia. Nature 586(7829):417–423. https://doi.org/10.1038/s41586-020-2777-8
Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75(5):762–777. https://doi.org/10.1016/j.neuron.2012.08.019
Nedergaard M (1994) Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science (New York, NY) 263(5154):1768–1771. https://doi.org/10.1126/science.8134839
Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH (2010) Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 224(1):123–132. https://doi.org/10.1016/j.expneurol.2010.03.002
Zündorf G, Kahlert S, Reiser G (2007) Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. J Neurochem 102(2):508–521. https://doi.org/10.1111/j.1471-4159.2007.04509.x
Haber M, Zhou L, Murai KK (2006) Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci 26(35):8881–8891. https://doi.org/10.1523/JNEUROSCI.1302-06.2006
Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT (2008) Neuron-glia interactions in the rat supraoptic nucleus. Prog Brain Res 170:109–117. https://doi.org/10.1016/S0079-6123(08)00410-X
Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468(7321):223–231. https://doi.org/10.1038/nature09612
Pannasch U, Freche D, Dallérac G, Ghézali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K, Abudara V, Dufour A, Lübke JH, Déglon N, Knott G, Holcman D, Rouach N (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17(4):549–558. https://doi.org/10.1038/nn.3662
Ullian EM, Christopherson KS, Barres BA (2004) Role for glia in synaptogenesis. Glia 47(3):209–216. https://doi.org/10.1002/glia.20082
Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science (New York, NY) 291(5504):657–661. https://doi.org/10.1126/science.291.5504.657
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10):3276–3287. https://doi.org/10.1523/JNEUROSCI.4707-08.2009
Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24(21):5016–5021. https://doi.org/10.1523/JNEUROSCI.0820-04.2004
Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94(4):1077–1098. https://doi.org/10.1152/physrev.00041.2013
Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13(5):584–591. https://doi.org/10.1038/nn.2535
Pósfai B, Cserép C, Orsolits B, Dénes Á (2019) New insights into microglia-neuron interactions: a neuron’s perspective. Neuroscience 405:103–117. https://doi.org/10.1016/j.neuroscience.2018.04.046
Fu H, Zhao Y, Hu D, Wang S, Yu T, Zhang L (2020) Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis 11(7):528. https://doi.org/10.1038/s41419-020-2733-4
Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME, Vernoux N, Tremblay MÈ, Fuehrmann T, Shoichet MS, Lacroix S (2019) Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10(1):518. https://doi.org/10.1038/s41467-019-08446-0
Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG (2017) Deletion of the Fractalkine Receptor, CX3CR1, Improves Endogenous Repair, Axon Sprouting, and Synaptogenesis after Spinal Cord Injury in Mice. J Neurosci 37(13):3568–3587. https://doi.org/10.1523/JNEUROSCI.2841-16.2017
Bushong EA, Martone ME, Ellisman MH (2004) Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 22(2):73–86. https://doi.org/10.1016/j.ijdevneu.2003.12.008
Witcher MR, Kirov SA, Harris KM (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55(1):13–23. https://doi.org/10.1002/glia.20415
Allen NJ, Eroglu C (2017) Cell biology of astrocyte-synapse interactions. Neuron 96(3):697–708. https://doi.org/10.1016/j.neuron.2017.09.056
Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22(1):183–192. https://doi.org/10.1523/JNEUROSCI.22-01-00183.2002
Wong ST, Atkinson BA, Weaver LC (2000) Confocal microscopic analysis reveals sprouting of primary afferent fibres in rat dorsal horn after spinal cord injury. Neurosci Lett 296(2–3):65–68. https://doi.org/10.1016/s0304-3940(00)01601-3
O’Shea TM, Burda JE, Sofroniew MV (2017) Cell biology of spinal cord injury and repair. J Clin Investig 127(9):3259–3270. https://doi.org/10.1172/JCI90608
Siddall PJ, Xu CL, Floyd N, Keay KA (1999) C-fos expression in the spinal cord of rats exhibiting allodynia following contusive spinal cord injury. Brain Res 851(1–2):281–286. https://doi.org/10.1016/s0006-8993(99)02173-3
Anderson AJ, Cummings BJ, Cotman CW (1994) Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer’s disease: association with pathology. Exp Neurol 125(2):286–295. https://doi.org/10.1006/exnr.1994.1031
Morishita T, Yamashita A, Katayama Y, Oshima H, Nishizaki Y, Shijo K, Fukaya C, Yamamoto T (2011) Chronological changes in astrocytes induced by chronic electrical sensorimotor cortex stimulation in rats. Neurol Med Chir 51(7):496–502. https://doi.org/10.2176/nmc.51.496
Fields RD, Burnstock G (2006) Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7(6):423–436. https://doi.org/10.1038/nrn1928
Fields RD, Ni Y (2010) Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci Signal 3(142):ra73. https://doi.org/10.1126/scisignal.2001128
Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM (2013) Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19(6):773–777. https://doi.org/10.1038/nm.3162
Turovsky EA, Braga A, Yu Y, Esteras N, Korsak A, Theparambil SM, Hadjihambi A, Hosford PS, Teschemacher AG, Marina N, Lythgoe MF, Haydon PG, Gourine AV (2020) Mechanosensory signaling in astrocytes. J Neurosci 40(49):9364–9371. https://doi.org/10.1523/JNEUROSCI.1249-20.2020
Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278(2):1354–1362. https://doi.org/10.1074/jbc.M209454200
Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40(5):971–982. https://doi.org/10.1016/s0896-6273(03)00717-7
Fields RD (2011) Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol 22(2):214–219. https://doi.org/10.1016/j.semcdb.2011.02.009
Imura Y, Morizawa Y, Komatsu R, Shibata K, Shinozaki Y, Kasai H, Moriishi K, Moriyama Y, Koizumi S (2013) Microglia release ATP by exocytosis. Glia 61(8):1320–1330. https://doi.org/10.1002/glia.22517
Tashima R, Koga K, Sekine M, Kanehisa K, Kohro Y, Tominaga K, Matsushita K, Tozaki-Saitoh H, Fukazawa Y, Inoue K, Yawo H, Furue H, Tsuda M (2018) Optogenetic activation of non-nociceptive Aβ fibers induces neuropathic pain-like sensory and emotional behaviors after nerve injury in rats. eNeuro. https://doi.org/10.1523/ENEURO.0450-17.2018
Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, Lombroso PJ, Salter MW (2016) Potentiation of synaptic GluN2B NMDAR currents by fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep 17(10):2753–2765. https://doi.org/10.1016/j.celrep.2016.11.024
Inoue K, Tsuda M (2009) Microglia and neuropathic pain. Glia 57(14):1469–1479. https://doi.org/10.1002/glia.20871
Ji RR, Xu ZZ, Gao YJ (2014) Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13(7):533–548. https://doi.org/10.1038/nrd4334
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28(44):11263–11268. https://doi.org/10.1523/JNEUROSCI.2308-08.2008
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438(7070):1017–1021. https://doi.org/10.1038/nature04223
Keller AF, Beggs S, Salter MW, De Koninck Y (2007) Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain 3:27. https://doi.org/10.1186/1744-8069-3-27
Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424(6951):938–942. https://doi.org/10.1038/nature01868
Tsuda M (2016) Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 7(1):17–26. https://doi.org/10.1111/jdi.12379
Teng Y, Zhang Y, Yue S, Chen H, Qu Y, Wei H, Jia X (2019) Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X4R in spinal cord microglia. J Neuroinflamm 16(1):271. https://doi.org/10.1186/s12974-019-1631-0
Karu TI, Kolyakov SF (2005) Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23(4):355–361. https://doi.org/10.1089/pho.2005.23.355
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8):607–610. https://doi.org/10.1002/iub.359
Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66(6):866–871. https://doi.org/10.1111/j.1751-1097.1997.tb03239.x
Passarella S (1989) He-Ne laser irradiation of isolated mitochondria. J Photochem Photobiol B 3(4):642–643. https://doi.org/10.1016/1011-1344(89)80090-9
Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D (2014) Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal 20(5):733–746. https://doi.org/10.1089/ars.2013.5229
Zupin L, Barbi E, Sagredini R, Ottaviani G, Crovella S, Celsi F (2021) In vitro effects of photobiomodulation therapy on 50B11 sensory neurons: evaluation of cell metabolism, oxidative stress, mitochondrial membrane potential (MMP), and capsaicin-induced calcium flow. J Biophotonics 14(2):e202000347. https://doi.org/10.1002/jbio.202000347
Cho H, Jeon HJ, Park S, Park CS, Chung E (2020) Neurite growth of trigeminal ganglion neurons in vitro with near-infrared light irradiation. J Photochem Photobiol 210:111959. https://doi.org/10.1016/j.jphotobiol.2020.111959
Acknowledgements
We thank Japan Medical Communications for the English-language proofreading. We also thank Y. Furuno and N. Saeki for supporting the experiments. This paper is dedicated to the memory of Dr. Hirofumi Sugano, who passed away on April 1, 2019.
Funding
This study was supported by JSPS KAKENHI Grant Number JP19K09527, JP22K09248, AMED/Research Project on Elucidation of Chronic Pain Grant Number JP20ek0610017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures were performed in accordance with the guidelines of the Laboratory Animals Care and Use Committee (No. No. 29025012, 20073, 21053, 22029). Efforts were made to minimize the number of animals used and to limit their suffering.
Consent to participate
Not applicable.
Consent to publication
All authors reviewed the draft and approved the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4510_MOESM1_ESM.tif
Supplementary file1 Fig. S1. Characterization of allodynia at the injury-level segment in SCI. (A–G) The positive response rate at the injury-level segment at each stimulation strength in the sham and 25 and 50 kdyn SCI groups. Sham, n=6; 25 kdyn, n=9; 50 kdyn, n=7. Error bars represent the mean±SD. (TIF 1096 kb)
18_2022_4510_MOESM2_ESM.tif
Supplementary file2 Fig. S2. Characterization of allodynia below the injury-site level in SCI. (A–G) The positive response rate below the injury level at each stimulation strength in the sham and 25 and 50 kdyn SCI groups. Sham, n=6; 25 kdyn, n=9; 50 kdyn, n=7. Error bars represent the mean±SD. (TIF 1081 kb)
18_2022_4510_MOESM3_ESM.tif
Supplementary file3 Fig. S3. Intracellular ATP at the injury site at three weeks after SCI. (A) The motor behavior in allodynia-negative and allodynia-positive mice. Sham, n=5; allodynia-negative, n=5; allodynia-positive, n=5. (B) Representative intracellular ATP images at 3 weeks after SCI. The maximum OFP/GFP value at 3.0 and the minimum value at 1.0. The right and left panels show the allodynia-negative and allodynia-positive groups, respectively. There was no significant difference between the allodynia-negative and allodynia-positive groups (p=0.137). Allodynia-negative, n=3; Allodynia-positive n=3. (C) A metabolomics analysis was conducted for the spinal cord segment at the injury site at three weeks after SCI. ATP, ADP, AMP, IMP. The tissue segmental ATP level did not differ between markedly the allodynia-negative and allodynia-positive groups. Allodynia-negative, n=3; Allodynia-positive n=3. Error bars represent the mean±SD. (TIF 24681 kb)
Supplementary file4 Supplementary movie 1. Positive response at the injury-level segment on the von Frey test. von Frey filaments were applied perpendicularly to the inferior half of the abdomen. Brisk withdrawal, shaking, lifting, or licking of the testing paw or abdomen were confirmed as positive responses. (MOV 16929 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakajima, N., Ohnishi, Y., Yamamoto, M. et al. Excess intracellular ATP causes neuropathic pain following spinal cord injury. Cell. Mol. Life Sci. 79, 483 (2022). https://doi.org/10.1007/s00018-022-04510-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04510-z