Abstract
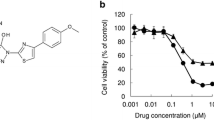
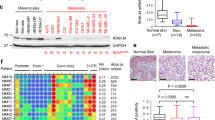
Melanoma is the most aggressive among all types of skin cancers. The current strategies against melanoma utilize BRAFV600E, as a focal point for targeted therapy. However, therapy resistance developed in melanoma patients against the conventional anti-melanoma drugs hinders the ultimate benefits of targeted therapies. A major mechanism by which melanoma cells attain therapy resistance is via the activation of microphthalmia-associated transcription factor-M (MITF-M), the key transcription factor and oncogene aiding the survival of melanoma cells. We demonstrate that tryptanthrin (Tpn), an indole quinazoline alkaloid, which we isolated and characterized from Wrightia tinctoria, exhibits remarkable anti-tumor activity towards human melanoma through the down-regulation of MITF-M. Microarray analysis of Tpn-treated melanoma cells followed by a STRING protein association network analysis revealed that differential expression of genes in melanoma converges at MITF-M. Furthermore, in vitro and in vivo studies conducted using melanoma cells with differential MITF-M expression status, endogenously or ectopically, demonstrated that the anti-melanoma activity of Tpn is decisively contingent on its efficacy in down-regulating MITF-M expression. Tpn potentiates the degradation of MITF-M via the modulation of MEK1/2-ERK1/2-MITF-M signaling cascades. Murine models demonstrate the efficacy of Tpn in attenuating the migration and metastasis of melanoma cells, while remaining pharmacologically safe. In addition, Tpn suppresses the expression of mutated BRAFV600E and inhibits Casein Kinase 2α, a pro-survival enzyme that regulates ERK1/2 homeostasis in many tumor types, including melanoma. Together, we point to a promising anti-melanoma drug in Tpn, by virtue of its attributes to impede melanoma invasion and metastasis by attenuating MITF-M.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Liu Y, Sheikh MS (2014) Melanoma: molecular pathogenesis and therapeutic management. Mol Cell Pharmacol 6:228
Bai X, Fisher DE, Flaherty KT (2019) Cell-state dynamics and therapeutic resistance in melanoma from the perspective of MITF and IFNγ pathways. Nat Rev Clin Oncol 16:549–562
Shackelford R, Pollen M, Vora M, Jusion TT, Cotelingam J, Nair B (2014) Malignant melanoma with concurrent BRAF E586K and NRAS Q81K mutations. Case Rep Oncol 7:297–300
Tsao H, Chin L, Garraway LA, Fisher DE (2012) Melanoma: from mutations to medicine. Genes Dev 26:1131–1155
Wellbrock C, Arozarena I (2016) The complexity of the ERK/MAP-kinase pathway and the treatment of melanoma skin cancer. Front Cell Dev Biol 4:33
Kim A, Cohen MS (2016) The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin Drug Discov 11:907–916
Holderfield M, Nagel T, Stuart D (2014) Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. Br J Cancer 111:640–645
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468:968–972
Hartman ML, Czyz M (2015) Pro-survival role of MITF in melanoma. J Investig Dermatol 135:352–358
Hartman ML, Czyz M (2015) MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci 72:1249–1260
Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J (2005) Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436:117–122
Cheli Y, Guiliano S, Botton T, Rocchi S, Hofman V, Hofman P, Bahadoran P, Bertolotto C, Ballotti R (2011) Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 30:2307–2318
Kido K, Sumimoto H, Asada S, Okada SM, Yaguchi T, Kawamura N, Miyagishi M, Saida T, Kawakami Y (2009) Simultaneous suppression of MITF and BRAFV600E enhanced inhibition of melanoma cell proliferation. Cancer Sci 100:1863–1869
Wellbrock C, Marais R (2005) Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation. J Cell Biol 170:703–708
Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, Nishimura EK, Golub TR, Fisher DE (2004) Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6:565–576
Johansson CH, Azimi A, Stolt MF, Shojaee S, Wiberg H, Grafström E, Hansson J, Brage SE (2013) Association of MITF and other melanosome-related proteins with chemoresistance in melanoma tumors and cell lines. Melanoma Res 23:360–365
Smith MP, Wellbrock C (2016) Molecular pathways: maintaining MAPK inhibitor sensitivity by targeting nonmutational tolerance. Clin Cancer Res 22:5966–5970
Antony J, Saikia M, Nath L, Katiki MR, Murty M, Paul A, Chandran H, Joseph SM, Panakkal EJ, Ran S (2015) DW-F5: a novel formulation against malignant melanoma from Wrightia tinctoria. Sci Rep 5:1–15
Shankar GM, Alex VV, Nisthul AA, Bava SV, Sundaram S, Retnakumari AP, Chittalakkottu S, Anto RJ (2020) Pre-clinical evidences for the efficacy of tryptanthrin as a potent suppressor of skin cancer. Cell Prolif 53:e12710
Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S (1998) Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280:1262–1265
Castelli M, Camps M, Gillieron C, Leroy D, Arkinstall S, Rommel C, Nichols A (2004) MAP kinase phosphatase 3 (MKP3) interacts with and is phosphorylated by protein kinase CK2α. J Biol Chem 279:44731–44739
Borgo C, Ruzzene M (2019) Role of protein kinase CK2 in antitumor drug resistance. J Exp Clin Cancer Res 38:1–15
Alchab F, Ettouati L, Bouaziz Z, Bollacke A, Delcros J-G, Gertzen CG, Gohlke H, Pinaud N, Marchivie M, Guillon J (2015) Synthesis, biological evaluation and molecular modeling of substituted indeno [1, 2-b] indoles as inhibitors of human protein kinase CK2. Pharmaceuticals 8:279–302
Bollacke A, Nienberg C, Le Borgne M, Jose J (2016) Toward selective CK2alpha and CK2alpha’inhibitors: development of a novel whole-cell kinase assay by autodisplay of catalytic CK2alpha’. J Pharm Biomed Anal 121:253–260
Thiel A, Moza M, Kytölä S, Orpana A, Jahkola T, Hernberg M, Virolainen S, Ristimäki A (2015) Prospective immunohistochemical analysis of BRAF V600E mutation in melanoma. Hum Pathol 46:169–175
Alcalá AM, Flaherty KT (2012) BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res 18:33–39
Salama AK, Flaherty KT (2013) BRAF in melanoma: current strategies and future directions. Clin Cancer Res 19:4326–4334
Ji Z, Flaherty KT, Tsao H (2012) Targeting the RAS pathway in melanoma. Trends Mol Med 18:27–35
Welsh SJ, Rizos H, Scolyer RA, Long GV (2016) Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer 62:76–85
Kataoka M, Hirata K, Kunikata T, Ushio S, Iwaki K, Ohashi K, Ikeda M, Kurimoto M (2001) Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol 36:5–9
Kaur R, Manjal SK, Rawal RK, Kumar K (2017) Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg Med Chem 25:4533–4552
Miao S, Shi X, Zhang H, Wang S, Sun J, Hua W, Miao Q, Zhao Y, Zhang C (2011) Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro. Int J Mol Sci 12:3831–3845
Yu S-T, Chen T-M, Tseng S-Y, Chen Y-H (2007) Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem Biophys Res Commun 358:79–84
Zeng Q, Luo C, Cho J, Lai D, Shen X, Zhang X, Zhou W (2021) Tryptanthrin exerts anti-breast cancer effects both in vitro and in vivo through modulating the inflammatory tumor microenvironment. Acta Pharm 71:245–266
Levy C, Khaled M, Fisher DE (2006) MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12:406–414
Aida S, Sonobe Y, Tanimura H, Oikawa N, Yuhki M, Sakamoto H, Mizuno T (2017) MITF suppression improves the sensitivity of melanoma cells to a BRAF inhibitor. Cancer Lett 409:116–124
Smith MP, Brunton H, Rowling EJ, Ferguson J, Arozarena I, Miskolczi Z, Lee JL, Girotti MR, Marais R, Levesque MP (2016) Inhibiting drivers of non-mutational drug tolerance is a salvage strategy for targeted melanoma therapy. Cancer Cell 29:270–284
Switzer B, Puzanov I, Skitzki JJ, Hamad L, Ernstoff MS (2022) Managing metastatic melanoma in 2022: a clinical review. JCO Oncol Pract 2022:OP. 21.00686
Rose AA, Annis MG, Frederick DT, Biondini M, Dong Z, Kwong L, Chin L, Keler T, Hawthorne T, Watson IR (2016) MAPK pathway inhibitors sensitize BRAF-mutant melanoma to an antibody-drug conjugate targeting GPNMB. Clin Cancer Res 22:6088–6098
Arozarena I, Smith MP, Wellbrock C (2016) Targeting MITF in the tolerance-phase. Oncotarget 7:54094
Kundu A, Quirit JG, Khouri MG, Firestone GL (2017) Inhibition of oncogenic BRAF activity by indole-3-carbinol disrupts microphthalmia-associated transcription factor expression and arrests melanoma cell proliferation. Mol Carcinog 56:49–61
Yokoyama S, Feige E, Poling LL, Levy C, Widlund HR, Khaled M, Kung AL, Fisher DE (2008) Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res 21:457–463
Faloon PW, Bennion M, Weiner WS, Smith RA, Wurst J, Weiwer M, Hartland C, Mosher CM, Johnston S, Porubsky P (2014) A small molecule inhibitor of the MITF molecular pathway. Probe reports from the NIH Molecular Libraries Program [Internet] 2014
Joshi SS, Hornyak TJ (2020) Cellular phenotypic plasticity of cutaneous melanoma: a complex puzzle. J Investig Dermatol 140:743–745
Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R (2008) Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS ONE 3:e2734
Kim Y, Rice AE, Denu JM (2003) Intramolecular dephosphorylation of ERK by MKP3. Biochemistry 42:15197–15207
Acknowledgements
The Department of Biotechnology supported Lifetime imaging facility at RGCB is acknowledged for the development of genetically encoded cell probes and fluorescence imaging. The authors are grateful to Dr.T.R.Santosh Kumar, Scientist G, RGCB, and Dr. Shine V.J., Post-Doctoral Researcher, RGCB for the help rendered in conducting the mt-roGFP assay. The authors acknowledge the technical help rendered by Ms. Rayginia P.Tennyson, Ms. Keerthana.C.K. and Mr. Prakash. R. The authors thank the veterinarians, Dr. Archana.S, Dr. Arya Aravind, and Dr. Vishnu Sunil Jaikumar, and all the personnel of the Animal research facility for the help rendered during the animal experiments. This work was financially supported by research grants from the Department of Biotechnology, Government of India (Ruby John Anto) and the Council for Scientific and Industrial Research, Government of India (Anwar Shabna, Jayesh Antony, and Vijayakurup Vinod).
Funding
This work was financially supported by research grants from Department of Biotechnology, Government of India and Council for Scientific and Industrial Research, Government of India.
Author information
Authors and Affiliations
Contributions
RJA conceived and supervised the study. The design and methodology of the study was developed by RJA, JA, AS and VV. Material preparation, data collection and analysis were performed by AS, JA, VV, MS, VBL, RP, NA, CS, DR, SJ, AVV, MS, SUA, USU, SS and SR. Review and editing of the manuscript was performed by NPA, VV, JA and SVB. All authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript and have given consent for publishing the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All the animal studies were done in accordance with the protocols approved by the Institutional Animal Ethics Committee (IAEC), (IAEC/298/RUBY/2015, IAEC/299/RUBY/2015, IAEC/300/RUBY/2015, IAEC/192/RUBY/2012, IAEC/696/RUBY/2018) Rajiv Gandhi Centre for Biotechnology, Department of Biotechnology, Government of India.
Consent to participate
Not applicable as human subjects were not involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shabna, A., Antony, J., Vijayakurup, V. et al. Pharmacological attenuation of melanoma by tryptanthrin pertains to the suppression of MITF-M through MEK/ERK signaling axis. Cell. Mol. Life Sci. 79, 478 (2022). https://doi.org/10.1007/s00018-022-04476-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04476-y