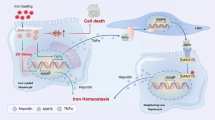
Abstract
Iron is an essential micro-element, involved in multiple biological activities in vertebrates. Excess iron accumulation has been identified as an important mediator of lipid deposition. However, the underlying mechanisms remain unknown. In the present study, we found that a high-iron diet significantly increased intestinal iron content and upregulated the mRNA expression of two iron transporters (zip14 and fpn1). Intestinal iron overload increased lipogenesis, reduced lipolysis and promoted oxidative stress and mitochondrial dysfunction. Iron-induced lipid accumulation was mediated by hypoxia-inducible factor-1 α (HIF1α), which was induced in response to mitochondrial oxidative stress following inhibition of prolyl hydroxylase 2 (PHD2). Mechanistically, iron promoted lipid deposition by enhancing the DNA binding capacity of HIF1α to the pparγ and fas promoters. Our results provide experimental evidence that oxidative stress, mitochondrial dysfunction and the HIF1α-PPARγ pathway are critical mediators of iron-induced lipid deposition.










Similar content being viewed by others
Data availability
All materials and data supporting this study are available from the corresponding author (luozhi99@mail.hzau.edu.cn) upon reasonable request.
Abbreviations
- 6PGD:
-
6-Phosphogluconate dehydrogenase
- ACCα:
-
Acetyl-CoA carboxylase α
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- ATP:
-
Adenosine triphosphate
- B2M:
-
Beta-2 microglobulin
- CAT:
-
Catalase
- DFO:
-
Deferoxamine mesylate
- DMEM:
-
Dulbecco's Modified Eagles Medium
- DMT1:
-
Divalent metal transporter 1
- ELFA:
-
Translation elongation factor
- EMSA:
-
Electrophoretic mobility-shift assay
- FAS:
-
Fatty acid synthase
- Fe:
-
Iron
- FBS:
-
Fetal bovine serum
- FPN1:
-
Ferroportin
- FerM:
-
Ferritin middle chain
- FTH:
-
Ferritin heavy chain
- FTL:
-
Ferritin light chain
- G6PD:
-
Glucose 6-phosphate dehydrogenase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT1:
-
Solute carrier family 2 member 1
- HK1:
-
Hexokinase 1
- HK2:
-
Hexokinase 2
- GK:
-
Glucokinase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- HIF1α:
-
Hypoxia-inducible factors-1α
- HIF2α:
-
Hypoxia-inducible factor-2α
- HPRT:
-
Hypoxanthine-guanine phosphoribosyltransferase
- HRE:
-
Hypoxia response elements
- HSL:
-
Hormone-sensitive lipase
- LDH:
-
Lactate dehydrogenase
- ICDH:
-
Isocitrate dehydrogenase
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- JC-1:
-
5,5ʹ,6,6ʹ-Tetrachloro-1,1ʹ,3,3ʹ-tetraethyl-imidacarbocyanine iodide
- LOX:
-
Lipoxygenase
- LPCAT3:
-
Lysophosphatidylcholine acyltransferase 3
- MDA:
-
Malondialdehyde
- ME:
-
Malic enzyme
- MMP:
-
Mitochondrial membrane potential
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- mtROS:
-
Mitochondrial ROS
- NEFA:
-
Nonesterified fatty acid
- NOX1:
-
NADPH oxidases 1
- NRF2:
-
Nuclear factor E2-related factor 2
- ORO:
-
Oil red O
- PFK:
-
Phosphofructokinase
- PGK1:
-
Phosphoglycerate kinase 1
- PHD2:
-
Prolyl hydroxylases 2
- PK:
-
Pyruvate kinase
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- PTGS2:
-
Prostaglandin endoperoxide synthase 2
- ROS:
-
Reactive oxygen species
- RPL7:
-
Ribosomal protein L7
- SEM:
-
Standard error of means
- SLC7A11:
-
Cystine-glutamate antiporter
- SREBP1:
-
Sterol regulatory element-binding proteins 1
- TEM:
-
Transmission electron microscopy
- TF:
-
Transferrin
- TFR1:
-
Transferrin receptor 1
- TFR2:
-
Transferrin receptor 2
- TG:
-
Triglyceride
- T-SOD:
-
Total superoxide dismutase
- TUBA:
-
Tubulin alpha chain
- UBCE:
-
Ubiquitin-conjugating enzyme
- UTR:
-
Untranslated region
- ZIP14:
-
ZRT-IRE-like protein 14
References
Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434:365–381
Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93:1721–1741
Kim CH, Leitch HA (2021) Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit Rev in Oncol Hematol 163:103367
Katsarou A, Pantopoulos K (2020) Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med 75:100866
Olivares-Rubio HF, Vega-López A (2016) Fatty acid metabolism in fish species as a biomarker for environmental monitoring. Environ Pollut 218:297–312
Xu YH, Hogstrand C, Xu YC, Zhao T, Zheng H, Luo Z (2021) Environmentally relevant concentrations of oxytetracycline and copper increased liver lipid deposition through inducing oxidative stress and mitochondria dysfunction in grass carp Ctenopharyngodon idella. Environ Pollut 283:117079
Ko SH, Kim HS (2020) Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 12:202
Ahmed U, Latham PS, Oates PS (2012) Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol 18:4651–4658
Choi JS, Koh IU, Lee HJ, Kim WH, Song J (2013) Effects of excess dietary iron and fat on glucose and lipid metabolism. J Nutr Biochem 24:1634–1644
Luo Z, Zou GY, Gao Y, Ye HM, Xi WQ, Liu X (2017) Effect of dietary iron (Fe) levels on growth performance, hepatic lipid metabolism and antioxidant responses in juvenile yellow catfish Pelteobagrus fulvidraco. Aquac Nutr 23:1475–1482
Fuqua BK, Vulpe CD, Anderson GJ (2012) Intestinal iron absorption. J Trace Elem Med Biol 26:115–119
Ko CW, Qu J, Black DD, Tso P (2020) Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol 17:169–183
Mansouri A, Gattolliat CH, Asselah T (2018) Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155:629–647
Tataranni T, Agriesti F, Mazzoccoli C, Ruggieri V, Scrima R, Laurenzana I, D’Auria F, Falzetti F, Di Ianni M, Musto P, Capitanio N, Piccoli C (2015) The iron chelator deferasirox affects redox signalling in haematopoietic stem/progenitor cells. Br J Haematol 170:236–246
Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N (2020) The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys 680:108241
Feng Z, Min L, Chen H, Deng W, Tan M, Liu H, Hou J (2021) Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol 43:101984
Pan YX, Zhuo MQ, Wei CC, Chen GH, Song YF, Luo Z (2018) Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol 199:12–20
Zhang DG, Zhao T, Hogstrand C, Ye HM, Xu XJ, Luo Z (2021) Oxidized fish oils increased lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the CREB1-Bcl2-Beclin1 pathway in the liver tissues and hepatocytes of yellow catfish. Food Chem 360:129814
Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W (2009) Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9:512–524
Marin J, Lozano E, Perez MJ (2016) Lack of mitochondrial DNA impairs chemical hypoxia-induced autophagy in liver tumor cells through ROS-AMPK-ULK1 signaling dysregulation independently of HIF-1α. Free Radic Biol Med 101:71–84
Lee P, Chandel NS, Simon MC (2020) Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol 21:268–283
Jia Y, Guo Y, Jin Q, Qu H, Qi D, Song P, Zhang X, Wang X, Xu W, Dong Y, Liang Y, Quan C (2020) A SUMOylation-dependent HIF-1α/CLDN6 negative feedback mitigates hypoxia-induced breast cancer metastasis. J Exp Clin Cancer Res 39:42
Luo J, Zhang X, He S, Lou Q, Zhai G, Shi C, Yin Z, Zheng F (2020) Deletion of narfl leads to increased oxidative stress mediated abnormal angiogenesis and digestive organ defects in zebrafish. Redox Biol 28:101355
Rankin EB, Giaccia AJ (2016) Hypoxic Control of Metastasis. Science 352:175–180
Fang W, Chen Q, Cui K, Chen Q, Li X, Xu N, Mai K, Ai Q (2021) Lipid overload impairs hepatic VLDL secretion via oxidative stress-mediated PKCδ-HNF4α-MTP pathway in large yellow croaker (Larimichthys crocea). Free Radic Biol Med 172:213–225
Zhao T, Wu K, Hogstrand C, Xu YH, Chen GH, Wei CC, Luo Z (2020) Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell Mol Life Sci 77:1987–2003
Chen GH, Song CC, Pantopoulos K, Wei XL, Zheng H, Luo Z (2022) Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic Biol Med 180:95–107
Wu K, Hogstrand C, Chen GH, Wei CC, Li DD, Luo Z (2018) Zn stimulates the phospholipids biosynthesis via the pathways of oxidative and endoplasmic reticulum stress in the intestine of freshwater teleost yellow catfish. Environ Sci Technol 52:9206–9214
Ling SC, Wu K, Zhang DG, Luo Z (2019) Endoplasmic reticulum stress-mediated autophagy and apoptosis alleviate dietary fat-induced triglyceride accumulation in the intestine and in isolated intestinal epithelial cells of yellow catfish. J Nutr 149:1732–1741
Dyer WJ, Bligh EG (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Song CC, Chen GH, Zhong CC, Chen F, Chen SW, Luo Z (2021) Transcriptional responses of four slc30a/znt family members and their roles in Zn homeostatic modulation in yellow catfish Pelteobagrus fulvidraco. Biochim Biophys Acta Gene Regul Mech 1864:194723
Zhong CC, Zhao T, Hogstrand C, Chen F, Song CC, Luo Z (2021) Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem 100:108883
Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, Campbell S, Welford SM (2017) HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat Commun 8:1769
Wu K, Tan XY, Xu YH, Chen GH, Zhuo MQ (2018) Functional analysis of promoters of genes in lipid metabolism and their transcriptional response to STAT3 under leptin signals. Genes 9:334
Chen GH, Luo Z, Chen F, Shi X, Song YF, You WJ, Liu X (2017) PPARα, PPARγ and SREBP-1 pathways mediated waterborne iron (Fe)-induced reduction in hepatic lipid deposition of javelin goby Synechogobius hasta. Comp Biochem Physiol C 197:8–18
Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD (2013) ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica 98:1049–1057
Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103:13612–13617
Jenkitkasemwong S, Wang CY, Coffey R, Zhang W, Chan A, Biel T, Kim JS, Hojyo S, Fukada T, Knutson MD (2015) SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab 22:138–150
Zhao N, Zhang AS, Worthen C, Knutson MD, Enns CA (2014) An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proc Natl Acad Sci USA 111:9175–9180
Katsarou A, Gkouvatsos K, Fillebeen C, Pantopoulos K (2021) Tissue-specific regulation of ferroportin in wild-type and Hjv-/- mice following dietary iron manipulations. Hepatol Commun 5:2139–2150
Iyengar V, Pullakhandam R, Nair KM (2012) Coordinate expression and localization of iron and zinc transporters explain iron-zinc interactions during uptake in Caco-2 cells: implications for iron uptake at the enterocyte. J Nutr Biochem 23:1146–1154
Marra F, Svegliati-Baroni G (2018) Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 68:280–295
Ertunc ME, Hotamisligil GS (2016) Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res 57:2099–2114
Sun X, Li X, Jia H, Wang H, Shui G, Qin Y, Shu X, Wang Y, Dong J, Liu G, Li X (2020) Nuclear factor E2-related factor 2 mediates oxidative stress-induced lipid accumulation in adipocytes by increasing adipogenesis and decreasing lipolysis. Antioxid Redox Signal 32:173–192
Galaris D, Barbouti A, Pantopoulos K (2019) Iron homeostasis and oxidative stress: an intimate relationship. Biochim Et Biophys Acta Mol Cell Res 1866:118535
Kim SH, Kim H (2018) Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 10:1137
Khamseekaew J, Kumfu S, Wongjaikam S, Kerdphoo S, Jaiwongkam T, Srichairatanakool S, Fucharoen S, Chattipakorn SC, Chattipakorn N (2017) Effects of iron overload, an iron chelator and a T-Type calcium channel blocker on cardiac mitochondrial biogenesis and mitochondrial dynamics in thalassemic mice. Eur J Pharmacol 799:118–127
Watts ER, Walmsley SR (2019) Inflammation and Hypoxia: HIF and PHD isoform selectivity. Trends Mol Med 25:33–46
Hu J, Meng F, Hu X, Huang L, Liu H, Liu Z, Li L (2020) Iron overload regulate the cytokine of mesenchymal stromal cells through ROS/HIF-1α pathway in Myelodysplastic syndromes. Leuk Res 93:106354
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol cell 30:393–402
Belanger AJ, Luo Z, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C (2007) Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun 364:567–572
Li D, Du Y, Yuan X, Han X, Dong Z, Chen X, Wu H, Zhang J, Xu L, Han C, Zhang M, Xia Q (2017) Hepatic hypoxia-inducible factors inhibit PPARα expression to exacerbate acetaminophen induced oxidative stress and hepatotoxicity. Free Radic Biol Med 110:102–116
Zhao YZ, Liu XL, Shen GM, Ma YN, Zhang FL, Chen MT, Zhao HL, Yu J, Zhang JW (2014) Hypoxia induces peroxisome proliferator-activated receptor γ expression via HIF-1-dependent mechanisms in HepG2 cell line. Arch Biochem Biophys 543:40–47
Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68:1003–1011
Acknowledgements
This work was supported by the National Key R&D Program of China (grant No. 2018YFD0900400).
Funding
This work was supported by the National Key R&D Program of China (grant No. 2018YFD0900400).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: CCS and ZL designed the experiments; CCS conducted the experiments and sample analyses with the help of GHC, CCZ, TZ, and DGZ; KP provided many critical suggestions for the experimental designs and data analysis; CCS analyzed the data and drafted the manuscript; KP and ZL revised the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors disclosed no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, CC., Pantopoulos, K., Chen, GH. et al. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1α-PPARγ pathway. Cell. Mol. Life Sci. 79, 394 (2022). https://doi.org/10.1007/s00018-022-04423-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04423-x