Abstract
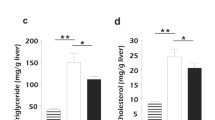
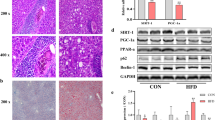
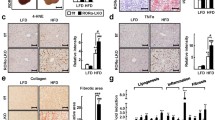
Non-alcoholic fatty liver disease (NAFLD) is related to a dysregulation of mitophagy, a process that is not fully understood. Parkin-related mitophagy can sustain mitochondrial homeostasis and hepatocyte viability. Herein, we report that selenoprotein M (SELENOM) plays a central role in maintaining mitophagy in high-fat diet (HFD)-mediated NAFLD. We show that SELENOM was significantly downregulated in the liver of HFD-fed mice. SELENOM deletion aggravated HFD-mediated hepatic steatosis, inflammation, and fibrosis; accompanied by enhanced fatty acid oxidation and oxidative stress in the liver. Molecular analyses show that lipotoxicity was related to increased mitochondrial apoptosis as evidenced by enhanced mitochondrial ROS production, and attenuation of mitochondrial potential in the liver of HFD-fed SELENOM−/− mice. Additionally, SELENOM deletion reduced mitophagy and aggravated hepatic injury in NAFLD. Mechanistically, SELENOM overexpression activated Parkin-mediated mitophagy to reduce mitochondrial apoptosis and remove HFD-damaged mitochondria. We further found that SELENOM regulates Parkin expression via the AMPKα1–MFN2 pathway; blockade of AMPKα1 prevented SELENOM activation of Parkin-mediated mitophagy. Our work identified SELENOM downregulation as a possible explanation for the defective mitophagy in NAFLD. Thus, targeting SELENOM may be potential new therapeutic modalities for NAFLD treatment.








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Polyzos SA, Kountouras J, Goulas A, Duntas L (2020) Selenium and selenoprotein P in nonalcoholic fatty liver disease. Hormones (Athens) 19:61–72. https://doi.org/10.1007/s42000-019-00127-3
Qiao L, Men L, Yu S et al (2022) Hepatic deficiency of selenoprotein S exacerbates hepatic steatosis and insulin resistance. Cell Death Dis 13:275. https://doi.org/10.1038/s41419-022-04716-w
Guerriero E, Accardo M, Capone F, Colonna G, Castello G, Costantini S (2014) Assessment of the Selenoprotein M (SELM) over-expression on human hepatocellular carcinoma tissues by immunohistochemistry. Eur J Histochem 58:2433. https://doi.org/10.4081/ejh.2014.2433
Zhou JC, Zhao H, Tang JY, Li JG, Liu XL, Zhu YM (2011) Molecular cloning, chromosomal localization and expression profiling of porcine selenoprotein M gene. Genes Genomics 33:529. https://doi.org/10.1007/s13258-010-0127-1
Gong T, Hashimoto AC, Sasuclark AR, Khadka VS, Gurary A, Pitts MW (2019) Selenoprotein M Promotes Hypothalamic Leptin Signaling and Thioredoxin Antioxidant Activity. Antioxid Redox Signal. https://doi.org/10.1089/ars.2018.7594
Ferguson AD, Labunskyy VM, Fomenko DE et al (2006) NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J Biol Chem 281:3536–3543. https://doi.org/10.1074/jbc.M511386200
Chen P, Wang RR, Ma XJ, Liu Q, Ni JZ (2013) Different forms of Selenoprotein M differentially affect Aβ aggregation and ROS generation. Int J Mol Sci 14:4385–4399. https://doi.org/10.3390/ijms14034385
Reeves MA, Bellinger FP, Berry MJ (2010) The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid Redox Signal 12:809–818. https://doi.org/10.1089/ars.2009.2883
Addinsall AB, Wright CR, Andrikopoulos S, van der Poel C, Stupka N (2018) Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem J 475:1037–1057. https://doi.org/10.1042/bcj20170920
Wang P, Lu Z, He M, Shi B, Lei X, Shan A (2020) The effects of endoplasmic-reticulum-resident Selenoproteins in a nonalcoholic fatty liver disease pig model induced by a high-fat diet. Nutrients. https://doi.org/10.3390/nu12030692
Ma YM, Guo YZ, Ibeanu G et al (2017) Overexpression of selenoprotein H prevents mitochondrial dynamic imbalance induced by glutamate exposure. Int J Biol Sci 13:1458–1469. https://doi.org/10.7150/ijbs.21300
Day K, Seale LA, Graham RM, Cardoso BR (2021) Selenotranscriptome network in non-alcoholic fatty liver disease. Front Nutr 8:744825. https://doi.org/10.3389/fnut.2021.744825
Pitts MW, Reeves MA, Hashimoto AC et al (2013) Deletion of selenoprotein M leads to obesity without cognitive deficits. J Biol Chem 288:26121–26134. https://doi.org/10.1074/jbc.M113.471235
Musso G, Cassader M, Paschetta E, Gambino R (2018) Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic Steatohepatitis. Gastroenterology 155:282–302. https://doi.org/10.1053/j.gastro.2018.06.031 (e288)
Ortiz M, Soto-Alarcón SA, Orellana P et al (2020) Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig Liver Dis 52:895–904. https://doi.org/10.1016/j.dld.2020.04.019
Nageeb MM, Khatab MI, Abdel-Sameea AA, Teleb NA (2020) Adelmidrol protects against non-alcoholic steatohepatitis in mice. Naunyn Schmiedebergs Arch Pharmacol 393:777–784. https://doi.org/10.1007/s00210-019-01785-1
Aqel B, DiBaise JK (2015) Role of the Gut microbiome in nonalcoholic fatty liver disease. Nutr Clin Pract 30:780–786. https://doi.org/10.1177/0884533615605811
Simões ICM, Fontes A, Pinton P, Zischka H, Wieckowski MR (2018) Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol 95:93–99. https://doi.org/10.1016/j.biocel.2017.12.019
Zhao H, Wang Y, Liu Y et al (2021) ROS-induced hepatotoxicity under Cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-κB/iκB-α pathways regulated by proteasome. Environ Sci Technol 55:6171–6183. https://doi.org/10.1021/acs.est.1c00515
Palikaras K, Lionaki E, Tavernarakis N (2015) Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521:525–528. https://doi.org/10.1038/nature14300
Qiu YN, Wang GH, Zhou F et al (2019) PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol Environ Saf 167:178–187. https://doi.org/10.1016/j.ecoenv.2018.08.050
Fan RF, Tang KK, Wang ZY, Wang L (2021) Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology 464:152999. https://doi.org/10.1016/j.tox.2021.152999
Wu M, Chen P, Liu F et al (2021) ONX0912, a selective oral proteasome inhibitor, triggering mitochondrial apoptosis and mitophagy in liver cancer. Biochem Biophys Res Commun 547:102–110. https://doi.org/10.1016/j.bbrc.2021.02.037
Yamada T, Murata D, Adachi Y et al (2018) Mitochondrial stasis reveals p62-mediated ubiquitination in Parkin-independent Mitophagy and Mitigates Nonalcoholic fatty liver disease. Cell Metab 28:588-604.e585. https://doi.org/10.1016/j.cmet.2018.06.014
Wang H, Ni HM, Chao X et al (2019) Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol 22:101148. https://doi.org/10.1016/j.redox.2019.101148
He L, Zhou Q, Huang Z et al (2019) PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKα and exacerbates atherosclerotic lesions. J Cell Physiol 234:8668–8682. https://doi.org/10.1016/j.ceb.2015.01.002
Hu Y, Chen H, Zhang L et al (2021) The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy 17:1142–1156. https://doi.org/10.1080/15548627.2020.1749490
Seabright AP, Fine NHF, Barlow JP et al (2020) AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. Faseb J 34:6284–6301. https://doi.org/10.1096/fj.201903051R
Zhang R, Chu K, Zhao N et al (2019) Corilagin alleviates nonalcoholic fatty liver disease in high-fat diet-induced C57BL/6 mice by ameliorating oxidative stress and restoring autophagic flux. Front Pharmacol 10:1693. https://doi.org/10.3389/fphar.2019.01693
Kumar S, Verma AK, Rani R et al (2020) Hepatic deficiency of augmenter of liver regeneration predisposes to nonalcoholic Steatohepatitis and fibrosis. Hepatology 72:1586–1604. https://doi.org/10.1002/hep.31167
Wan J, Zhang Y, Yang D et al (2021) Gastrodin improves nonalcoholic fatty liver disease via activation of the AMPK signaling pathway. Hepatology. https://doi.org/10.1002/hep.32068
Zhang Y, Xu Y, Chen B, Zhao B, Gao XJ (2021) Selenium deficiency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02882-0
Song N, Wang W, Wang Y, Guan Y, Xu S, Guo MY (2021) Hydrogen sulfide of air induces macrophage extracellular traps to aggravate inflammatory injury via the regulation of miR-15b-5p on MAPK and insulin signals in trachea of chickens. Sci Total Environ 771:145407. https://doi.org/10.1016/j.scitotenv.2021.145407
Momcilovic M, Shirihai O, Murphy MP, Koehler CM, Sadeghi S, Shackelford DB (2020) Reply to: in vivo quantification of mitochondrial membrane potential. Nature 583:E19-e20. https://doi.org/10.1038/s41586-020-2367-9
Miao Z, Miao Z, Shi X, Wu H, Yao Y, Xu S (2022) The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotoxicol Environ Saf 231:113176. https://doi.org/10.1016/j.ecoenv.2022.113176
Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, Gladyshev VN (2007) Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem 282:11960–11968. https://doi.org/10.1074/jbc.M701605200
Zheng Y, Guan H, Yang J, Cai J, Liu Q, Zhang Z (2021) Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr 7:997–1008. https://doi.org/10.21203/rs.3.rs-118703/v1
Chi Q, Hu X, Liu Z, Han Y, Li S (2021) H2S exposure induces cell death in the broiler thymus via the ROS-initiated JNK/MST1/FOXO1 pathway. Ecotoxicol Environ Saf 222:112488. https://doi.org/10.1016/j.ecoenv.2021.112488
Liu XJ, Wang YQ, Shang SQ, Xu S, Guo M (2022) TMT induces apoptosis and necroptosis in mouse kidneys through oxidative stress-induced activation of the NLRP3 inflammasome. Ecotoxicol Environ Saf 230:113167. https://doi.org/10.1016/j.ecoenv.2022.113167
Schuster S, Cabrera D, Arrese M, Feldstein AE (2018) Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 15:349–364. https://doi.org/10.1038/s41575-018-0009-6
Chen X, Bi M, Yang J et al (2022) Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater 421:126704. https://doi.org/10.1016/j.jhazmat.2021.126704
Sangineto M, Bukke VN, Bellanti F et al (2021) A novel nutraceuticals mixture improves liver Steatosis by preventing oxidative stress and mitochondrial dysfunction in a NAFLD model. Nutrients. https://doi.org/10.3390/nu13020652
Zhang HR, Zhao FQ, Gai XX et al (2022) Astilbin attenuates apoptosis induced by cadmium through oxidative stress in carp(Cyprinus carpio L.) head kidney lymphocyte. Fish Shellfish Immunol 13:S1050–4648. https://doi.org/10.1016/j.fsi.2022.05.021
Paech F, Abegg VF, Duthaler U, Terracciano L, Bouitbir J, Krähenbühl S (2018) Sunitinib induces hepatocyte mitochondrial damage and apoptosis in mice. Toxicology 409:13–23. https://doi.org/10.1016/j.tox.2018.07.009
Kruppa AJ, Buss F (2018) Actin cages isolate damaged mitochondria during mitophagy. Autophagy 14:1644–1645. https://doi.org/10.1080/15548627.2018.1486152
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30:678–688. https://doi.org/10.1016/j.molcel.2008.06.001
Eiyama A, Okamoto K (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 33:95–101. https://doi.org/10.1016/j.ceb.2015.01.002
Park HS, Song JW, Park JH et al (2020) TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy. https://doi.org/10.1080/15548627.2020.1834711
Gong T, Hashimoto AC, Sasuclark AR, Khadka VS, Gurary A, Pitts MW (2021) Selenoprotein M promotes hypothalamic Leptin signaling and Thioredoxin antioxidant activity. Antioxid Redox Signal 35:775–787. https://doi.org/10.1089/ars.2018.7594
Zhang ZH, Song GL (2021) Roles of Selenoproteins in brain function and the potential mechanism of selenium in Alzheimer’s disease. Front Neurosci 15:646518. https://doi.org/10.3389/fnins.2021.646518
Qazi IH, Yang H, Wei S et al (2020) Dietary selenium deficiency and supplementation differentially modulate the expression of two ER-resident selenoproteins (selenoprotein K and selenoprotein M) in the ovaries of aged mice: preliminary data. Reprod Biol 20:441–446. https://doi.org/10.1016/j.repbio.2020.07.006
Gong T, Berry MJ, Pitts MW (2016) Selenoprotein M: structure, expression and functional relevance. Springer International Publishing. https://doi.org/10.1007/978-3-319-41283-2_21
Varlamova EG et al (2019) Protein partners of Selenoprotein SELM and the role of selenium compounds in regulation of its expression in human cancer cells. Dokl Biochem Biophys 488:300–303. https://doi.org/10.1134/S1607672919050065
Liu Q, Du P, Zhu Y, Zhang X, Cai J, Zhang Z (2022) Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol Life Sci 79:106
Li J, Zhang W, Zhou P, Tong X, Guo D, Lin H (2022) Selenium deficiency induced apoptosis via mitochondrial pathway caused by Oxidative Stress in porcine gastric tissues. Res Vet Sci 144:142–148. https://doi.org/10.1134/S1607672919050065
Chi Q, Luan Y, Zhang Y, Hu X, Li S (2019) The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics 11:845–857. https://doi.org/10.1039/c9mt00006b
Gilkerson RW, De Vries RL, Lebot P et al (2012) Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet 21:978–990. https://doi.org/10.1093/hmg/ddr529
Miao Z, Miao Z, Wang S, Wu H, Xu S (2022) Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol 120:674–685. https://doi.org/10.1016/j.fsi.2021.12.017
Tanaka M, Sato A, Kishimoto Y, Mabashi-Asazuma H, Kondo K, Iida K (2020) Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients. https://doi.org/10.3390/nu12051479
Tanaka S, Hikita H, Tatsumi T et al (2016) Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 64:1994–2014. https://doi.org/10.1002/hep.28820
Reinartz A, Ehling J, Leue A et al (2010) Lipid-induced up-regulation of human acyl-CoA synthetase 5 promotes hepatocellular apoptosis. Biochim Biophys Acta 1801:1025–1035. https://doi.org/10.1016/j.bbalip.2010.04.010
Wen F, Shi Z, Liu X et al (2021) Acute elevated resistin exacerbates mitochondrial damage and aggravates liver Steatosis through AMPK/PGC-1α signaling pathway in male NAFLD mice. Horm Metab Res 53:132–144. https://doi.org/10.1055/a-1293-8250
Singh R, Kaushik S, Wang Y et al (2009) Autophagy regulates lipid metabolism. Nature 458:1131–1135. https://doi.org/10.1038/nature07976
Dong H, Zhou W, Xin J et al (2019) Salvinorin A moderates postischemic brain injury by preserving endothelial mitochondrial function via AMPK/Mfn2 activation. Exp Neurol 322:113045. https://doi.org/10.1016/j.expneurol.2019.113045
Lyons CL, Roche HM (2018) Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms19103092
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023. https://doi.org/10.1038/ncb2329
Funding
This study was supported by the National Natural Science Foundation of China (31872531).
Author information
Authors and Affiliations
Contributions
JC designed the study and JH wrote the manuscript. JY is the guarantor of this work. XC, HZ and YZ researched data and helped design experiments. QL and ZZ takes responsibility for the integrity of the data and helped design experiments. All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Journal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Animal experiments in our study were subject to ethical review by the Animal Care and Use Committee of Northeast Agricultural University (SRM-11), Harbin, China. The investigation conforms to directive 2010/63/EU of the European parliament. All authors agreed to participate.
Consent for publication
All authors read the manuscript and agreed to submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, J., Huang, J., Yang, J. et al. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: the role of the AMPKα1–MFN2 pathway and Parkin mitophagy. Cell. Mol. Life Sci. 79, 354 (2022). https://doi.org/10.1007/s00018-022-04385-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04385-0