Abstract
Retinal degeneration (RD) is recognized as a frequent cause of visual impairments, including inherited (Retinitis pigmentosa) and degenerative (age-related macular) eye diseases. Dental stem cells (DSCs) have recently demonstrated a promising neuroprotection potential for ocular diseases through a paracrine manner carried out by extracellular vesicles (EVs). However, effective isolation of EVs is still challenging, and isolation methods determine the composition of enriched EVs and the subsequent biological and functional effects. In the present study, we assessed two enrichment methods (micro-electromechanical systems and ultrafiltration) to isolate the EVs from stem cells from apical papilla (SCAP). The size distribution of the corresponding isolates exhibited the capability of each method to enrich different subsets of EVs, which significantly impacts their biological and functional effects. We confirmed the neuroprotection and anti-inflammatory capacity of the SCAP-EVs in vitro. Further experiments revealed the possible therapeutic effects of subretinal injection of SCAP-EVs in the Royal College of Surgeons (RCS) rat model. We found that EVs enriched by the micro-electromechanical-based device (MEMS-EVs) preserved visual function, reduced retinal cell apoptosis, and prevented thinning of the outer nuclear layer (ONL). Interestingly, the effect of MEMS-EVs was extended to the retinal ganglion cell/retinal nerve fiber layer (GCL/RNFL). This study supports the use of the microfluidics approach to enrich valuable subsets of EVs, together with the choice of SCAP as a source to derive EVs for cell-free therapy of RD.

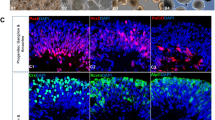
source to derive EVs. a Bright-field (phase contrast) image of homogenous spindle-shaped SCAP, b Electron micrograph of SCAP ultrastructure; arrows show MVB containing EVs, c sEVs: small (30–110 nm), d m/lEVs: medium to large (100–1000 nm) (yellow arrow), e Experimental workflow of conditioned medium collection, f Electron micrograph of conditioned medium indicating a heterogeneous population of EVs (yellow arrow), g Flow cytometry analysis; the area under the pink line identifies EVs reacting with CD81. The area under the yellow line indicates the interactions of EVs with corresponding non-reactive immunoglobulin of the same isotype. h Western blot results of SCAP conditioned medium. SCAP stem cells from apical papilla, EV extracellular vesicles, MVB multivesicular bodies, CM conditioned medium





Similar content being viewed by others
References
Ding SSL, Subbiah SK, Khan MSA et al (2019) Empowering mesenchymal stem cells for ocular degenerative disorders. Int J Mol Sci 20:1784
Adak S, Magdalene D, Deshmukh S et al (2021) A review on mesenchymal stem cells for treatment of retinal diseases. Stem Cell Rev Reports 17:1154–1173
Luo L, He Y, Wang X et al (2018) Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. https://doi.org/10.1155/2018/1731289
Kolar MK, Itte VN, Kingham PJ et al (2017) The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-12969-1
De Almeida JFA, Chen P, Henry MA, Diogenes A (2014) Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng—Part A 20:3089–3100. https://doi.org/10.1089/ten.tea.2013.0347
Mead B, Logan A, Berry M et al (2014) Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. https://doi.org/10.1371/journal.pone.0109305
Mead B, Logan A, Berry M et al (2013) Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig Ophthalmol Vis Sci 54:7544–7556. https://doi.org/10.1167/iovs.13-13045
Sonoyama W, Liu Y, Yamaza T et al (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171. https://doi.org/10.1016/j.joen.2007.11.021
Yu S, Zhao Y, Ma Y, Ge L (2016) Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev 25:499–508. https://doi.org/10.1089/scd.2015.0298
Karamali F, Esfahani MHN, Taleahmad S et al (2018) Stem cells from apical papilla promote differentiation of human pluripotent stem cells towards retinal cells. Differentiation 101:8–15. https://doi.org/10.1016/j.diff.2018.02.003
Kang J, Fan W, Deng Q et al (2019) Stem cells from the apical papilla: a promising source for stem cell-based therapy. Biomed Res Int. https://doi.org/10.1155/2019/6104738
Volarevic V, Markovic BS, Gazdic M et al (2018) Ethical and safety issues of stem cell-based therapy. Int J Med Sci 15:36–45
Yu B, Li XR, Zhang XM (2020) Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J Stem Cells 12:178–187. https://doi.org/10.4252/wjsc.v12.i3.178
Deng CL, Hu CB, Ling ST et al (2020) Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ 28:1041–1061. https://doi.org/10.1038/s41418-020-00636-4
Yang B, Chen Y, Shi J (2019) Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater 31:e1802896. https://doi.org/10.1002/adma.201802896
Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902
Théry C, Witwer KW, Aikawa E et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. https://doi.org/10.1080/20013078.2018.1535750
Bian B, Zhao C, He X et al (2020) Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J Extracell Vesicles 9:1748931. https://doi.org/10.1080/20013078.2020.1748931
Seyedrazizadeh SZ, Poosti S, Nazari A et al (2020) Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res Ther 11:203. https://doi.org/10.1186/s13287-020-01702-x
Mead B, Tomarev S (2017) Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through mirna-dependent mechanisms. Stem Cells Transl Med 6:1273–1285. https://doi.org/10.1002/sctm.16-0428
Huyen Phan T, Kamini Divakarla S, Hao Yeo J et al (2021) New multiscale characterisation methodology for effective determination of isolation-structure-function relationship of extracellular vesicles. BioRxiv. https://doi.org/10.1101/2021.02.09.430523
Lamparski HG, Metha-Damani A, Yao JY et al (2002) Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 270:211–226. https://doi.org/10.1016/S0022-1759(02)00330-7
Webber J, Clayton A (2013) How pure are your vesicles? J Extracell Vesicles. https://doi.org/10.3402/jev.v2i0.19861
Momen-Heravi F, Balaj L, Alian S et al (2013) Current methods for the isolation of extracellular vesicles. Biol Chem 394:1253–1262
Islam N, Sayed S (2012) MEMS Microfluidics for Lab-on-a-Chip Applications, In (Ed.). Microelectromechanical Systems and Devices, INTECH, London. https://doi.org/10.5772/39206
Christ RD, Wernli RL (2014) Navigational sensors. ROV Man. https://doi.org/10.1016/B978-0-08-098288-5.00017-8
Chen J, Xu Y, Wang X et al (2019) Rapid and efficient isolation and detection of extracellular vesicles from plasma for lung cancer diagnosis. Lab Chip 19:432–443. https://doi.org/10.1039/c8lc01193a
Ibsen SD, Wright J, Lewis JM et al (2017) Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano 11:6641–6651. https://doi.org/10.1021/acsnano.7b00549
Lewis JM, Vyas AD, Qiu Y et al (2018) Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano 12:3311–3320. https://doi.org/10.1021/acsnano.7b08199
Hadady H, Wong JJ, Hiibel SR et al (2014) High frequency dielectrophoretic response of microalgae over time. Electrophoresis. https://doi.org/10.1002/elps.201400306
Ramos A, Morgan H, Green NG, Castellanos A (1998) Ac electrokinetics: a review of forces in microelectrode structures. J Phys D Appl Phys 31:2338–2353
Peng Y, Tang L, Zhou Y (2017) Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res 58:217–226. https://doi.org/10.1159/000479157
Dureau P, Legat L, Neuner-Jehle M et al (2000) Quantitative analysis of subretinal injections in the rat. Graefe’s Arch Clin Exp Ophthalmol 238:608–614. https://doi.org/10.1007/s004170000156
Vezina M, Bussieres M, Glazier G, Gagnon MP, Martel D (2011) Determination of injectable intravitreous volumes in rats. Investigative Ophthalmol Visual Sci 52:3219–3219
Hadady H, Karamali F, Ejeian F et al (2021) AC electrokinetic isolation and detection of extracellular vesicles from dental pulp stem cells: theoretical simulation incorporating fluid mechanics. Electrophoresis. https://doi.org/10.1002/elps.202000376
Wang J, Bonacquisti EE, Brown AD, Nguyen J (2020) Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 9:660. https://doi.org/10.3390/cells9030660
Ben MK, Habeler W, Plancheron A et al (2017) Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aai7471
EJ van der V, EN N-’t H, W S, et al (2012) Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc 7:1311–1326. https://doi.org/10.1038/NPROT.2012.065
Tzameret A, Sher I, Edelstain V et al (2019) Evaluation of visual function in Royal College of Surgeon rats using a depth perception visual cliff test. Vis Neurosci. https://doi.org/10.1017/S095252381800007X
Yuan D, Zhao Y, Banks WA et al (2017) Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142:1–12. https://doi.org/10.1016/J.BIOMATERIALS.2017.07.011
Starikova S, Jones C, Forman WR et al (2016) A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J Phys Conf Ser 733:012039. https://doi.org/10.1088/1742-6596/733/1/012039
Tj H, Ta H, HK V, (2004) Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res 294:458–468. https://doi.org/10.1016/J.YEXCR.2003.11.016
Fan XL, Zhang Y, Li X, Fu QL (2020) Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 77:2771–2794
Andrukhov O, Behm C, Blufstein A, Rausch-Fan X (2019) Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells 11:604. https://doi.org/10.4252/WJSC.V11.I9.604
Liu XM, Liu Y, Yu S et al (2019) Potential immunomodulatory effects of stem cells from the apical papilla on Treg conversion in tissue regeneration for regenerative endodontic treatment. Int Endod J 52:1758–1767. https://doi.org/10.1111/IEJ.13197
Naseri MH, Mahdavi M, Davoodi J et al (2015) Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int 15:1–9. https://doi.org/10.1186/S12935-015-0204-2/FIGURES/4
Leibowitz B, Yu J (2010) Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther 9:417. https://doi.org/10.4161/CBT.9.6.11392
Stenvinkel P, Ketteler M, Johnson RJ et al (2005) IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67:1216–1233. https://doi.org/10.1111/J.1523-1755.2005.00200.X
Ryals RC, Andrews MD, Datta S et al (2017) Long-term characterization of retinal degeneration in royal college of surgeons rats using spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci 58:1378–1386. https://doi.org/10.1167/iovs.16-20363
Carr A-J, Vugler AA, Hikita ST et al (2009) Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One 4:e8152. https://doi.org/10.1371/journal.pone.0008152
Dai J, Fu Y, Zeng Y et al (2017) Improved retinal function in RCS rats after suppressing the over-activation of mGluR5. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-03702-z
Zeiss CJ, Johnson EA (2004) Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Invest Ophthalmol Vis Sci 45:971–976. https://doi.org/10.1167/IOVS.03-0301
Tan B, Barathi VA, Lin E et al (2020) Longitudinal structural and microvascular observation in RCS rat eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 61:54–54. https://doi.org/10.1167/IOVS.61.6.54
Wooff Y, Cioanca AV, Chu-Tan JA et al (2020) Small–medium extracellular vesicles and their miRNA cargo in retinal health and degeneration: mediators of homeostasis, and vehicles for targeted gene therapy. Front Cell Neurosci 14:160. https://doi.org/10.3389/FNCEL.2020.00160/BIBTEX
Mead B, Amaral J, Tomarev S (2018) Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Ophthalmol Vis Sci 59:702–714. https://doi.org/10.1167/IOVS.17-22855
Luzuriaga J, Polo Y, Pastor-alonso O et al (2021) Advances and perspectives in dental pulp stem cell based neuroregeneration therapies. Int J Mol Sci 22:3546
Abuarqoub D, Aslam N, Almajali B et al (2020) Neuro-regenerative potential of dental stem cells: a concise review. Cell Tissue Res 382:267–279
Imanishi Y, Hata M, Matsukawa R et al (2021) Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm Regen 41:12. https://doi.org/10.1186/s41232-021-00163-w
Kong F, Wu CT, Geng P et al (2021) Dental pulp stem cell-derived extracellular vesicles mitigate haematopoietic damage after radiation. Stem Cell Rev Reports 17:318–331. https://doi.org/10.1007/s12015-020-10020-x
Ke Y, Fan X, Hao R et al (2021) Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating Oct4 to promote retinal Müller cell retrodifferentiation via HSP90. Stem Cell Res Ther 12:21. https://doi.org/10.1186/s13287-020-02034-6
Woods J, Pellegrino J, Burch J (2011) Generalized guidance for considering pore-size distribution in membrane distillation. J Memb Sci 368:124–133. https://doi.org/10.1016/j.memsci.2010.11.041
Brennan K, Martin K, FitzGerald SP et al (2020) (2020) A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 101(10):1–13. https://doi.org/10.1038/s41598-020-57497-7
Mol EA, Goumans MJ, Doevendans PA et al (2017) Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed Nanotech Biol Med 13:2061–2065. https://doi.org/10.1016/J.NANO.2017.03.011
Cho S, Jo W, Heo Y et al (2016) Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens Actuat B Chem 233:289–297. https://doi.org/10.1016/J.SNB.2016.04.091
Wenzel A, Grimm C, Samardzija M, Remé CE (2005) Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res 24:275–306
Noailles A, Maneu V, Campello L et al (2016) Persistent inflammatory state after photoreceptor loss in an animal model of retinal degeneration. Sci Rep 6:1–16. https://doi.org/10.1038/srep33356
Zhang S, Ye J, Dong G (2010) Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. J Mol Neurosci 40:311–320. https://doi.org/10.1007/s12031-009-9285-5
Bray AF, Cevallos RR, Gazarian K, Lamas M (2014) Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neuroscience 280:142–155. https://doi.org/10.1016/j.neuroscience.2014.09.023
Zeng XX, Ng YK, Ling EA (2000) Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci 17:463–471. https://doi.org/10.1017/S0952523800173122
Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P et al (2009) Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci 50:3860–3868. https://doi.org/10.1167/IOVS.08-3267
Johansson UE, Eftekhari S, Warfvinge K (2010) A battery of cell- and structure-specific markers for the adult porcine retina. J Histochem Cytochem 58:377–389. https://doi.org/10.1369/JHC.2009.954933
Heineck DP, Lewis JM, Heller MJ (2017) Electrokinetic device design and constraints for use in high conductance solutions. Electrophoresis 38:1475–1482. https://doi.org/10.1002/ELPS.201600563
Diaz-Armas GG, Cervantes-Gonzalez AP, Martinez-Duarte R, Perez-Gonzalez VH (2022) Electrically driven microfluidic platforms for exosome manipulation and characterization. Electrophoresis 43:327–339. https://doi.org/10.1002/ELPS.202100202
Pardue MT, Allen RS (2018) Neuroprotective strategies for retinal disease. Prog Retin Eye Res 65:50–76
Cabral T, Mello LGM, Lima LH et al (2017) Retinal and choroidal angiogenesis: a review of new targets. Int J Retin Vitr 3:31
Paulus YM, Sodhi A (2017) Anti-angiogenic therapy for retinal disease. Handbook of Experimental Pharmacology. Springer, New York, pp 271–307
Liu Y, Zhuang X, Yu S et al (2021) Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res Ther 12:1–14. https://doi.org/10.1186/s13287-021-02151-w
Bakopoulou A, Kritis A, Andreadis D et al (2015) Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments. Stem Cells Dev 24:2496–2512. https://doi.org/10.1089/scd.2015.0197
Acknowledgements
The authors wish to express their gratitude to Professor Hossein Baharvand and Dr. Faezeh Shekari for providing rhodopsin antibody and excellent technical assistance.
Funding
This study is based upon work supported by the Irans’ National Elites Foundation (INEF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics approval
Animal care and experimental procedures were performed in accordance with the Helsinki Declaration and compiled with the ARRIVE guideline (https://arriveguidelines.org/) and were approved by the institutional research ethics committee at Royan institute (IR.ACECR.ROYAN.REC.1400.043).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Data availability statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hadady, H., Karamali, F., Ejeian, F. et al. Potential neuroprotective effect of stem cells from apical papilla derived extracellular vesicles enriched by lab-on-chip approach during retinal degeneration. Cell. Mol. Life Sci. 79, 350 (2022). https://doi.org/10.1007/s00018-022-04375-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04375-2