Abstract
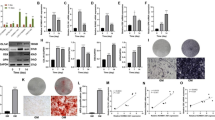
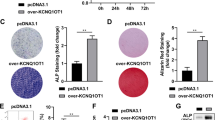
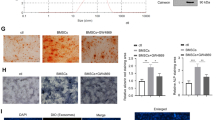
The osteogenic potential of bone marrow mesenchymal stem cells (BMSCs) is critical for bone formation and regeneration. A high non-/delayed-union rate of fracture healing still occurs in specific populations, implying an urgent need to discover novel targets for promoting osteogenesis and bone regeneration. Long non-coding (lnc)RNAs are emerging regulators of multiple physiological processes, including osteogenesis. Based on differential expression analysis of RNA sequencing data, we found that lncRNA AC132217.4, a 3'UTR-overlapping lncRNA of insulin growth factor 2 (IGF2), was highly induced during osteogenic differentiation of BMSCs. Afterward, both gain-of-function and loss-of-function experiments proved that AC132217.4 promotes osteoblast development from BMSCs. As for its molecular mechanism, we found that AC132217.4 binds with IGF2 mRNA to regulate its expression and downstream AKT activation to control osteoblast maturation and function. Furthermore, we identified two splicing factors, splicing component 35 KDa (SC35) and heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1), which regulate the biogenesis of AC132217.4 at the post-transcriptional level. We also identified a transcription factor, ALX1, which regulates AC132217.7 expression at the transcriptional level to promote osteogenesis. Importantly, in-vivo over-expression of AC132217.4 essentially promotes the bone healing process in a murine tibial drill-hole model. Our study demonstrates that lncRNA AC132217.4 is a novel anabolic regulator of BMSC osteogenesis and could be a plausible therapeutic target for improving bone regeneration.








Similar content being viewed by others
Data available statement
RNA-seq data of hBMSCs during osteogenesis are deposited in the NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE114117 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi). All other data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Donev R, Newall A, Thome J, Sheer D (2007) A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms. Mol Psychiatry 12(7):681–690. https://doi.org/10.1038/sj.mp.4001971
Munir H, McGettrick HM (2015) Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev 24(18):2091–2100. https://doi.org/10.1089/scd.2015.0008
Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Buscher D et al (2010) Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 69(1):241–248. https://doi.org/10.1136/ard.2008.101881
Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7(6):259–264. https://doi.org/10.1016/S1471-4914(01)02016-0
Abarrategi A, Mian SA, Passaro D, Rouault-Pierre K, Grey W, Bonnet D (2018) Modeling the human bone marrow niche in mice: from host bone marrow engraftment to bioengineering approaches. J Exp Med 215(3):729–743. https://doi.org/10.1084/jem.20172139
Wu M, Chen G, Li YP (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Gennari L, Rotatori S, Bianciardi S, Nuti R, Merlotti D (2016) Treatment needs and current options for postmenopausal osteoporosis. Expert Opin Pharmacother 17(8):1141–1152. https://doi.org/10.1080/14656566.2016.1176147
Black DM, Rosen CJ (2016) Clinical practice. Postmenopausal osteoporosis. N Engl J Med 374(3):254–262. https://doi.org/10.1056/NEJMcp1513724
Lin H, Sohn J, Shen H, Langhans MT, Tuan RS (2019) Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials 203:96–110. https://doi.org/10.1016/j.biomaterials.2018.06.026
Zhu G, Zhang T, Chen M, Yao K, Huang X, Zhang B, Li Y et al (2021) Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact Mater 6(11):4110–4140. https://doi.org/10.1016/j.bioactmat.2021.03.043
Chen G, Xu H, Yao Y, Xu T, Yuan M, Zhang X, Lv Z et al (2020) BMP signaling in the development and regeneration of cranium bones and maintenance of calvarial stem cells. Front Cell Dev Biol 8:135. https://doi.org/10.3389/fcell.2020.00135
Jiao H, Xiao E, Graves DT (2015) Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep 13(5):327–335. https://doi.org/10.1007/s11914-015-0286-8
Kline AJ, Gruen GS, Pape HC, Tarkin IS, Irrgang JJ, Wukich DK (2009) Early complications following the operative treatment of pilon fractures with and without diabetes. Foot Ankle Int 30(11):1042–1047. https://doi.org/10.3113/fai.2009.1042
Liu SJ, Dang HX, Lim DA, Feng FY, Maher CA (2021) Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. https://doi.org/10.1038/s41568-021-00353-1
Ulitsky I, Bartel David P (2013) lincRNAs: Genomics, evolution, and mechanisms. Cell 154(1):26–46. https://doi.org/10.1016/j.cell.2013.06.020
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15(1):7–21. https://doi.org/10.1038/nrg3606
Hung T, Wang YL, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM et al (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43(7):621-U196. https://doi.org/10.1038/ng.848
Gu H, Li Z, Lv XF, Zhao AB, Zhu MY, Zhang Y (2019) LncRNA KCNQ1OT1 delayed fracture healing through the Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci 23(11):4575–4583. https://doi.org/10.26355/eurrev_201906_18034
Li D, Liu J, Yang C, Tian Y, Yin C, Hu L, Chen Z et al (2021) Targeting long noncoding RNA PMIF facilitates osteoprogenitor cells migrating to bone formation surface to promote bone formation during aging. Theranostics 11(11):5585–5604. https://doi.org/10.7150/thno.54477
Liu YB, Lin LP, Zou R, Zhao QH, Lin FQ (2019) Silencing long non-coding RNA MEG3 accelerates tibia fraction healing by regulating the Wnt/β-catenin signalling pathway. J Cell Mol Med 23(6):3855–3866. https://doi.org/10.1111/jcmm.14229
Liu ZC, Xu YL, Jiang Y, Liu Y, Wei ZC, Liu SG, Yang SJ (2019) Low-expression of lncRNA-ANCR promotes tibial fracture healing via targeting RUNX2. Eur Rev Med Pharmacol Sci 23(3 Suppl):60–66. https://doi.org/10.26355/eurrev_201908_18629
Wang L, Wang YP, Li ZY, Li ZQ, Yu B (2015) Differential expression of long noncoding ribonucleic acids during osteogenic differentiation of human bone marrow mesenchymal stem cells. Int Orthop 39(5):1013–1019. https://doi.org/10.1007/s00264-015-2683-0
Jia Q, Jiang WK, Ni LX (2015) Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Arch Oral Biol 60(2):234–241. https://doi.org/10.1016/j.archoralbio.2014.10.007
Nojima T, Proudfoot NJ (2022) Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev Mol Cell Biol. https://doi.org/10.1038/s41580-021-00447-6
Quinn JJ, Chang HY (2016) Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17(1):47–62. https://doi.org/10.1038/nrg.2015.10
Statello L, Guo CJ, Chen LL, Huarte M (2021) Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 22(2):96–118. https://doi.org/10.1038/s41580-020-00315-9
Li X, Ma C, Zhang L, Li N, Zhang X, He J, He R et al (2017) LncRNAAC132217.4, a KLF8-regulated long non-coding RNA, facilitates oral squamous cell carcinoma metastasis by upregulating IGF2 expression. Cancer Lett 407:45–56. https://doi.org/10.1016/j.canlet.2017.08.007
Zhang C, Hong FF, Wang CC, Li L, Chen JL, Liu F, Quan RF et al (2017) TRIB3 inhibits proliferation and promotes osteogenesis in hBMSCs by regulating the ERK1/2 signaling pathway. Sci Rep UK. https://doi.org/10.1038/s41598-017-10601-w
Li Z, Helms JA (2021) Drill hole models to investigate bone repair. Methods Mol Biol 2221:193–204. https://doi.org/10.1007/978-1-0716-0989-7_12
Chen EEM, Zhang W, Ye CYCY, Gao X, Jiang LJLJ, Zhao TFTF, Pan ZJZJ et al (2017) Knockdown of SIRT7 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly via activation of the Wnt/beta-catenin signaling pathway. Cell Death Dis. https://doi.org/10.1038/cddis.2017.429
Wang Y, Bikle DD, Chang W (2013) Autocrine and paracrine actions of IGF-I signaling in skeletal development. Bone Res 1(3):249–259. https://doi.org/10.4248/br201303003
Yakar S, Werner H, Rosen CJ (2018) Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol 61(1):T115-t137. https://doi.org/10.1530/jme-17-0298
Shi Y, Chen J, Karner CM, Long F (2015) Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation. Proc Natl Acad Sci USA 112(15):4678–4683. https://doi.org/10.1073/pnas.1502301112
Hardouin SN, Guo R, Romeo PH, Nagy A, Aubin JE (2011) Impaired mesenchymal stem cell differentiation and osteoclastogenesis in mice deficient for Igf2-P2 transcripts. Development (Cambridge, England) 138(2):203–213. https://doi.org/10.1242/dev.054916
Kang H, Sung J, Jung HM, Woo KM, Hong SD, Roh S (2012) Insulin-like growth factor 2 promotes osteogenic cell differentiation in the parthenogenetic murine embryonic stem cells. Tissue Eng Part A 18(3–4):331–341. https://doi.org/10.1089/ten.TEA.2011.0074
Einhorn TA, Gerstenfeld LC (2015) Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 11(1):45–54. https://doi.org/10.1038/nrrheum.2014.164
Liu SJ, Lim DA (2018) Modulating the expression of long non-coding RNAs for functional studies. EMBO Rep. https://doi.org/10.15252/embr.201846955
Ettensohn CA, Illies MR, Oliveri P, De Jong DL (2003) Alx1, a member of the Cart1/Alx3/Alx4 subfamily of paired-class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development (Cambridge, England) 130(13):2917–2928. https://doi.org/10.1242/dev.00511
Khor JM, Ettensohn CA (2017) Functional divergence of paralogous transcription factors supported the evolution of biomineralization in echinoderms. Elife. https://doi.org/10.7554/eLife.32728
Beverdam A, Brouwer A, Reijnen M, Korving J, Meijlink F (2001) Severe nasal clefting and abnormal embryonic apoptosis in Alx3/Alx4 double mutant mice. Development (Cambridge, England) 128(20):3975–3986
Dee CT, Szymoniuk CR, Mills PE, Takahashi T (2013) Defective neural crest migration revealed by a Zebrafish model of Alx1-related frontonasal dysplasia. Hum Mol Genet 22(2):239–251. https://doi.org/10.1093/hmg/dds423
Iyyanar PPR, Wu Z, Lan Y, Hu YC, Jiang R (2022) Alx1 deficient mice recapitulate craniofacial phenotype and reveal developmental basis of ALX1-related frontonasal dysplasia. Front Cell Dev Biol 10:777887. https://doi.org/10.3389/fcell.2022.777887
Lyons LA, Erdman CA, Grahn RA, Hamilton MJ, Carter MJ, Helps CR, Alhaddad H et al (2016) Aristaless-like homeobox protein 1 (ALX1) variant associated with craniofacial structure and frontonasal dysplasia in Burmese cats. Dev Biol 409(2):451–458. https://doi.org/10.1016/j.ydbio.2015.11.015
Zhao Q, Behringer RR, de Crombrugghe B (1996) Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet 13(3):275–283. https://doi.org/10.1038/ng0796-275
Pini J, Kueper J, Hu YD, Kawasaki K, Yeung P, Tsimbal C, Yoon B et al (2020) ALX1-related frontonasal dysplasia results from defective neural crest cell development and migration. EMBO Mol Med 12(10):e12013. https://doi.org/10.15252/emmm.202012013
Uz E, Alanay Y, Aktas D, Vargel I, Gucer S, Tuncbilek G, von Eggeling F et al (2010) Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal-recessive ALX-related frontonasal dysplasia. Am J Hum Genet 86(5):789–796. https://doi.org/10.1016/j.ajhg.2010.04.002
Masunaga Y, Inoue T, Yamoto K, Fujisawa Y, Sato Y, Kawashima-Sonoyama Y, Morisada N et al (2020) IGF2 mutations. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz034
Yamoto K, Saitsu H, Nakagawa N, Nakajima H, Hasegawa T, Fujisawa Y, Kagami M et al (2017) De novo IGF2 mutation on the paternal allele in a patient with Silver–Russell syndrome and ectrodactyly. Hum Mutat 38(8):953–958. https://doi.org/10.1002/humu.23253
Yang L, Li Y, Gong R, Gao MQ, Feng C, Liu TY, Sun Y et al (2019) The long non-coding RNA-ORLNC1 regulates bone mass by directing mesenchymal stem cell fate. Mol Ther 27(2):394–410. https://doi.org/10.1016/j.ymthe.2018.11.019
Chen LA, Jiang W, Huang JY, He BC, Zuo GW, Zhang WL, Luo Q et al (2010) Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res 25(11):2447–2459. https://doi.org/10.1002/jbmr.133〹
Ding W, Li J, Singh J, Alif R, Vazquez-Padron RI, Gomes SA, Hare JM et al (2015) miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovasc Res 106(1):131–142. https://doi.org/10.1093/cvr/cvv030
Hamidouche Z, Fromigue O, Ringe J, Haupl T, Marie PJ (2010) Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. https://doi.org/10.1186/1471-2121-11-44
Iyer S, Margulies BS, Kerr WG (2013) Role of SHIP1 in bone biology. Ann N Y Acad Sci 1280:11–14. https://doi.org/10.1111/nyas.12091
Guntur AR, Rosen CJ (2011) The skeleton: a multi-functional complex organ: new insights into osteoblasts and their role in bone formation: the central role of PI3Kinase. J Endocrinol 211(2):123–130. https://doi.org/10.1530/joe-11-0175
Sun MY, Chi GF, Xu JJ, Tan Y, Xu JY, Lv S, Xu ZR et al (2018) Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin alpha 5. Stem Cell Res Ther. https://doi.org/10.1186/s13287-018-0798-0
Andrew JG, Hoyland J, Freemont AJ, Marsh D (1993) Insulinlike growth factor gene expression in human fracture callus. Calcif Tissue Int 53(2):97–102. https://doi.org/10.1007/bf01321886
Koh A, Niikura T, Lee SY, Oe K, Koga T, Dogaki Y, Kurosaka M (2011) Differential gene expression and immunolocalization of insulin-like growth factors and insulin-like growth factor binding proteins between experimental nonunions and standard healing fractures. J Orthop Res 29(12):1820–1826. https://doi.org/10.1002/jor.21457
Chen QQ, Wang WM (2014) Expression of FGF-2 and IGF-1 in diabetic rats with fracture. Asian Pac J Trop Med 7(1):71–75. https://doi.org/10.1016/s1995-7645(13)60195-9
Szczęsny G, Olszewski WL, Zagozda M, Rutkowska J, Czapnik Z, Swoboda-Kopeć E, Górecki A (2011) Genetic factors responsible for long bone fractures non-union. Arch Orthop Trauma Surg 131(2):275–281. https://doi.org/10.1007/s00402-010-1171-7
Wang T, Wang Y, Menendez A, Fong C, Babey M, Tahimic CG, Cheng Z et al (2015) Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res 30(9):1572–1584. https://doi.org/10.1002/jbmr.2510
Taniguchi T, Matsumoto T, Shindo H (2003) Changes of serum levels of osteocalcin, alkaline phosphatase, IGF-I and IGF-binding protein-3 during fracture healing. Injury 34(7):477–479. https://doi.org/10.1016/s0020-1383(02)00380-7
Weiss S, Henle P, Bidlingmaier M, Moghaddam A, Kasten P, Zimmermann G (2008) Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Hormone IGF Res 18(3):205–212. https://doi.org/10.1016/j.ghir.2007.09.002
Bach LA (2015) Endothelial cells and the IGF system. J Mol Endocrinol 54(1):R1-13. https://doi.org/10.1530/jme-14-0215
Fowlkes JL, Thrailkill KM, Liu L, Wahl EC, Bunn RC, Cockrell GE, Perrien DS et al (2006) Effects of systemic and local administration of recombinant human IGF-I (rhIGF-I) on de novo bone formation in an aged mouse model. J Bone Miner Res 21(9):1359–1366. https://doi.org/10.1359/jbmr.060618
Zhang X, Xing H, Qi F, Liu H, Gao L, Wang X (2020) Local delivery of insulin/IGF-1 for bone regeneration: carriers, strategies, and effects. Nanotheranostics 4(4):242–255. https://doi.org/10.7150/ntno.46408
Wang F, Song YL, Li CX, Li DH, Zhang HP, Ma AJ, Xi XQ et al (2010) Sustained release of insulin-like growth factor-1 from poly(lactide-co-glycolide) microspheres improves osseointegration of dental implants in type 2 diabetic rats. Eur J Pharmacol 640(1–3):226–232. https://doi.org/10.1016/j.ejphar.2010.04.024
Locatelli V, Bianchi VE (2014) Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol 2014:235060. https://doi.org/10.1155/2014/235060
Majidinia M, Sadeghpour A, Yousefi B (2018) The roles of signaling pathways in bone repair and regeneration. J Cell Physiol 233(4):2937–2948. https://doi.org/10.1002/jcp.26042
Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken J, Tabata Y et al (2014) Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 35(31):8829–8839. https://doi.org/10.1016/j.biomaterials.2014.07.006
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (31900513, 81900806, 32070814), Application Program for Chinese Manned Space Station (YYWT-0901-EXP-06), and Qianjiang Talent Program of Zhejiang Province (QJD1902024).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, MW, JW and CZ; methodology, MW, JW and CZ; investigation, CZ, SW, EC; resources, MW and CZ writing—original draft, MW, SW and CZ; writing—review and editing, MW and CZ; funding acquisition, MW YL and CZ; supervision, MW and JW.
Corresponding authors
Ethics declarations
Conflict of interest
There are no competing financial interests related to the work described.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Wu, S., Chen, E. et al. ALX1-transcribed LncRNA AC132217.4 promotes osteogenesis and bone healing via IGF-AKT signaling in mesenchymal stem cells. Cell. Mol. Life Sci. 79, 328 (2022). https://doi.org/10.1007/s00018-022-04338-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04338-7