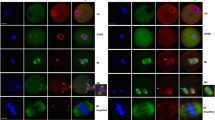
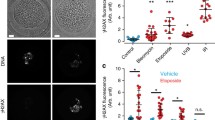
Abstract
Mammalian oocytes are particularly susceptible to accumulating DNA damage. However, unlike mitotic cells in which DNA damage induces G2 arrest by activating the ATM-Chk1/2-Cdc25 pathway, oocytes readily enter M-phase immediately following DNA damage. This implies a lack of a robust canonical G2/M DNA damage checkpoint in oocytes. Here we show that MDC1 plays a non-canonical role in controlling G2/M transition by regulating APC/C-Cdh1-mediated cyclin B1 degradation in response to DNA damage in mouse oocytes. Depletion of MDC1 impaired M-phase entry by decreasing cyclin B1 levels via the APC/C-Cdh1 pathway. Notably, the APC/C-Cdh1 regulation mediated by MDC1 was achieved by a direct interaction between MDC1 and APC/C-Cdh1. This interaction was transiently disrupted after DNA damage with a concomitant increase in Cdh1 levels, which, in turn, decreased cyclin B1 levels and delayed M-phase entry. Moreover, MDC1 depletion impaired spindle assembly by decreasing the integrity of microtubule organizing centers (MTOCs). Therefore, our results demonstrate that MDC1 is an essential molecule in regulating G2/M transition in response to DNA damage and in regulating spindle assembly in mouse oocytes. These results provide new insights into the regulation of the G2/M DNA damage checkpoint and cell cycle control in oocytes.






Similar content being viewed by others
Data availability
The authors confirmed that all data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19(2):238–245. https://doi.org/10.1016/j.ceb.2007.02.009
Blackford AN, Jackson SP (2017) ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell 66(6):801–817. https://doi.org/10.1016/j.molcel.2017.05.015
Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3(5):421–429. https://doi.org/10.1016/s1535-6108(03)00110-7
Lou Z, Minter-Dykhouse K, Wu X, Chen J (2003) MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421(6926):957–961. https://doi.org/10.1038/nature01447
Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ (2003) MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 421(6926):961–966. https://doi.org/10.1038/nature01446
Panier S, Boulton SJ (2014) Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 15(1):7–18. https://doi.org/10.1038/nrm3719
Polo SE, Jackson SP (2011) Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev 25(5):409–433. https://doi.org/10.1101/gad.2021311
Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP (2005) MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123(7):1213–1226. https://doi.org/10.1016/j.cell.2005.09.038
Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A (2003) Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 5(7):675–679. https://doi.org/10.1038/ncb1004
Han SJ, Chen R, Paronetto MP, Conti M (2005) Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 15(18):1670–1676. https://doi.org/10.1016/j.cub.2005.07.056
Morgan DO (1995) Principles of CDK regulation. Nature 374(6518):131–134. https://doi.org/10.1038/374131a0
Adhikari D, Busayavalasa K, Zhang J, Hu M, Risal S, Bayazit MB, Singh M, Diril MK, Kaldis P, Liu K (2016) Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan. Cell Res 26(11):1212–1225. https://doi.org/10.1038/cr.2016.119
Holt JE, Tran SM, Stewart JL, Minahan K, Garcia-Higuera I, Moreno S, Jones KT (2011) The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development 138(5):905–913. https://doi.org/10.1242/dev.059022
Reis A, Chang HY, Levasseur M, Jones KT (2006) APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol 8(5):539–540
Carroll J, Marangos P (2013) The DNA damage response in mammalian oocytes. Front Genet 4:117. https://doi.org/10.3389/fgene.2013.00117
Marangos P, Carroll J (2012) Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol 22(11):989–994. https://doi.org/10.1016/j.cub.2012.03.063
Collins JK, Jones KT (2016) DNA damage responses in mammalian oocytes. Reproduction 152(1):R15-22. https://doi.org/10.1530/REP-16-0069
Collins JK, Lane SIR, Merriman JA, Jones KT (2015) DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. Nat Commun 6:8553. https://doi.org/10.1038/ncomms9553
Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J (2015) DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun 6:8706. https://doi.org/10.1038/ncomms9706
Mayer A, Baran V, Sakakibara Y, Brzakova A, Ferencova I, Motlik J, Kitajima TS, Schultz RM, Solc P (2016) DNA damage response during mouse oocyte maturation. Cell Cycle 15(4):546–558. https://doi.org/10.1080/15384101.2015.1128592
Subramanian GN, Greaney J, Wei Z, Becherel O, Lavin M, Homer HA (2020) Oocytes mount a noncanonical DNA damage response involving APC-Cdh1-mediated proteolysis. J Cell Biol 219 (4). doi:https://doi.org/10.1083/jcb.201907213
Eliezer Y, Argaman L, Kornowski M, Roniger M, Goldberg M (2014) Interplay between the DNA damage proteins MDC1 and ATM in the regulation of the spindle assembly checkpoint. J Biol Chem 289(12):8182–8193. https://doi.org/10.1074/jbc.M113.532739
Barra V, Fachinetti D (2018) The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat Commun 9(1):4340. https://doi.org/10.1038/s41467-018-06545-y
Bai GY, Choe MH, Kim JS, Oh JS (2020) Mis12 controls cyclin B1 stabilization via Cdc14B-mediated APC/C(Cdh1) regulation during meiotic G2/M transition in mouse oocytes. Development 147 (8). doi:https://doi.org/10.1242/dev.185322
Homer H, Gui L, Carroll J (2009) A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 326(5955):991–994. https://doi.org/10.1126/science.1175326
Holt JE, Weaver J, Jones KT (2010) Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development 137(8):1297–1304. https://doi.org/10.1242/dev.047555
Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3(12):995–1000. https://doi.org/10.1038/nmeth947
Schuh M, Ellenberg J (2007) Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130(3):484–498. https://doi.org/10.1016/j.cell.2007.06.025
Coster G, Hayouka Z, Argaman L, Strauss C, Friedler A, Brandeis M, Goldberg M (2007) The DNA damage response mediator MDC1 directly interacts with the anaphase-promoting complex/cyclosome. J Biol Chem 282(44):32053–32064. https://doi.org/10.1074/jbc.M705890200
Townsend K, Mason H, Blackford AN, Miller ES, Chapman JR, Sedgwick GG, Barone G, Turnell AS, Stewart GS (2009) Mediator of DNA damage checkpoint 1 (MDC1) regulates mitotic progression. J Biol Chem 284(49):33939–33948. https://doi.org/10.1074/jbc.M109.009191
Krenning L, Feringa FM, Shaltiel IA, van den Berg J, Medema RH (2014) Transient activation of p53 in G2 phase is sufficient to induce senescence. Mol Cell 55(1):59–72. https://doi.org/10.1016/j.molcel.2014.05.007
Mullers E, Silva Cascales H, Jaiswal H, Saurin AT, Lindqvist A (2014) Nuclear translocation of Cyclin B1 marks the restriction point for terminal cell cycle exit in G2 phase. Cell Cycle 13(17):2733–2743. https://doi.org/10.4161/15384101.2015.945831
Wiebusch L, Hagemeier C (2010) p53- and p21-dependent premature APC/C-Cdh1 activation in G2 is part of the long-term response to genotoxic stress. Oncogene 29(24):3477–3489. https://doi.org/10.1038/onc.2010.99
Clift D, Schuh M (2015) A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun 6:7217. https://doi.org/10.1038/ncomms8217
Rai R, Phadnis A, Haralkar S, Badwe RA, Dai H, Li K, Lin SY (2008) Differential regulation of centrosome integrity by DNA damage response proteins. Cell Cycle 7(14):2225–2233. https://doi.org/10.4161/cc.7.14.6303
Namgoong S, Kim NH (2018) Meiotic spindle formation in mammalian oocytes: implications for human infertility. Biol Reprod 98(2):153–161. https://doi.org/10.1093/biolre/iox145
Joukov V, Walter JC, De Nicolo A (2014) The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol Cell 55(4):578–591. https://doi.org/10.1016/j.molcel.2014.06.016
Li Z, Shao C, Kong Y, Carlock C, Ahmad N, Liu X (2017) DNA Damage Response-Independent Role for MDC1 in Maintaining Genomic Stability. Molecular and cellular biology 37 (9). https://doi.org/10.1128/MCB.00595-16
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A03015642 and NRF-2019R1I1A2A01041413).
Author information
Authors and Affiliations
Contributions
JL and JSO conceived and designed the experiments. JL performed all experiments. JL and JSO analyzed and interpreted the data. JSO supervised the study. JL and JSO wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interests to declare.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leem, J., Oh, J.S. MDC1 is essential for G2/M transition and spindle assembly in mouse oocytes. Cell. Mol. Life Sci. 79, 200 (2022). https://doi.org/10.1007/s00018-022-04241-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04241-1