Abstract
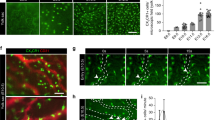
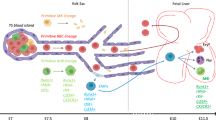
Yolk sac (YS) CSF1 receptor positive (CSF1R+) cells are thought to be the progenitors for tissue-resident macrophages present in various tissues. The YS progenitors for tissue-resident macrophages are referred to as erythroid–myeloid progenitors (EMPs). However, diverse types of hematopoietic progenitors are present in the early YS, thus it is not precisely known which type of hematopoietic cell gives rise to the CSF1R+ lineage. In this study, an analysis was conducted to determine when CSF1R+ progenitors appeared in the early YS. It showed that CSF1R+ cells appeared in the YS as early as embryonic day 9 (E9) and that the earliest hematopoietic progenitors that differentiate into CSF1R+ cells were found in E8. Since these progenitors possessed the capability to generate primitive erythroid cells, it was likely that primitive erythroid lineages shared progenitors with the CSF1R+ lineage. Mutual antagonism appears to work between PU.1 and GATA1 when CSF1R+ cells appear in the early YS. One day later (E9), multiple progenitors, including myeloid-restricted progenitors and multipotent progenitors, in the YS could immediately generate CSF1R+ cells. These results suggest that EMPs are not an exclusive source for the CSF1R+ lineage; rather, multiple hematopoietic cell populations give rise to CSF1R+ lineage in the early YS.





Similar content being viewed by others
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- YS:
-
Yolk sac
- E:
-
Embryonic day
- EMP:
-
Erythroid-myeloid progenitor
- CPDP:
-
Common primitive-definitive precursor
- MRP:
-
Myeloid-restricted progenitor
- BM:
-
Bone marrow
References
Unanue ER (1984) Antigen-presenting function of the macrophage. Annu Rev Immunol 2:395–428. https://doi.org/10.1146/annurev.iy.02.040184.002143
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14:986–995. https://doi.org/10.1038/ni.2705
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. https://doi.org/10.1126/science.1194637
Schulz C, Perdiguero EG, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90. https://doi.org/10.1126/science.1219179
Varol C, Mildner A, Jung S (2015) Macrophages: development and tissue specialization. Annu Rev Immunol 33:643–675. https://doi.org/10.1146/annurev-immunol-032414-112220
Blériot C, Chakarov S, Ginhoux F (2020) Determinants of resident tissue macrophage identity and function. Immunity 52:957–970. https://doi.org/10.1016/j.immuni.2020.05.014
Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I (2005) Three pathways to mature macrophages in the early mouse yolk sac. Blood 106:3004–3011. https://doi.org/10.1182/blood-2005-02-0461
Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547–551. https://doi.org/10.1038/nature13989
Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin A, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JKY, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F (2015) C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42:665–678. https://doi.org/10.1016/j.immuni.2015.03.011
Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA (2011) Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9:541–552. https://doi.org/10.1016/j.stem.2011.10.003
McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Palis J (2015) Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep 11:1892–1904. https://doi.org/10.1016/j.celrep.2015.05.036
Yamane T (2018) Mouse yolk sac hematopoiesis. Front Cell Dev Biol 6:80. https://doi.org/10.3389/fcell.2018.00080
Yamane T, Hosen N, Yamazaki H, Weissman IL (2009) Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci USA 106:8953–8958. https://doi.org/10.1073/pnas.0904090106
Ito C, Yamazaki H, Yamane T (2013) Earliest hematopoietic progenitors at embryonic day 9 preferentially generate B-1 B cells rather than follicular B or marginal zone B cells. Biochem Biophys Res Commun 437:307–313. https://doi.org/10.1016/j.bbrc.2013.06.073
Yamane T, Washino A, Yamazaki H (2013) Common developmental pathway for primitive erythrocytes and multipotent hematopoietic progenitors in early mouse development. Stem Cell Rep 1:590–603. https://doi.org/10.1016/j.stemcr.2013.10.008
Yamane T, Ito C, Washino A, Isono K, Yamazaki H (2017) Repression of primitive erythroid program is critical for the initiation of multi-lineage hematopoiesis in mouse development. J Cell Physiol 232:323–330. https://doi.org/10.1002/jcp.25422
Hayashi SI, Miyamoto A, Yamane T, Kataoka H, Ogawa M, Sugawara S, Nishikawa S, Nishikawa S, Sudo T, Yamazaki H, Kunisada T (1997) Osteoclast precursors in bone marrow and peritoneal cavity. J Cell Physiol 170:241–247. https://doi.org/10.1002/(SICI)1097-4652(199703)170:3%3c241::AID-JCP4%3e3.0.CO;2-O
Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M (2013) Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 14:821–830. https://doi.org/10.1038/ni.2638
Okabe Y, Medzhitov R (2014) Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157:832–844. https://doi.org/10.1016/j.cell.2014.04.016
Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR (2014) The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344:645–648. https://doi.org/10.1126/science.1251414
Saeys Y, Van Gassen S, Lambrecht BN (2016) Computational flow cytometry: helping to make sense of high-dimensional immunology data. Nat Rev Immunol 16:449–462. https://doi.org/10.1038/nri.2016.56
Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, Rauner M, Yadav VK, Crozet L, Bohm M, Loyher PL, Karsenty G, Waskow C, Geissmann F (2019) Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 568:541–545. https://doi.org/10.1038/s41586-019-1105-7
Yahara Y, Barrientos T, Tang YJ, Puviindran V, Nadesan P, Zhang H, Gibson JR, Gregory SG, Diao Y, Xiang Y, Qadri YJ, Souma T, Shinohara ML, Alman BA (2020) Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat Cell Biol 22:49–59. https://doi.org/10.1038/s41556-019-0437-8
Rekhtman N, Radparvar F, Evans T, Skoultchi AI (1999) Direct interaction of hematopoietic transcription factors PU. 1 and GATA-1: functional antagonism in erythroid cells. Genes Dev 13:1398–1411. https://doi.org/10.1101/gad.13.11.1398
Nerlov C, Querfurth E, Kulessa H, Graf T (2000) GATA-1 interacts with the myeloid PU. 1 transcription factor and represses PU. 1-dependent transcription. Blood 95:2543–2551. https://doi.org/10.1182/blood.V95.8.2543
Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, Narravula S, Torbett BE, Orkin SH, Tenen DG (2000) PU. 1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96:2641–2648. https://doi.org/10.1182/blood.V96.8.2641
Wontakal SN, Guo X, Smith C, MacCarthy T, Bresnick EH, Bergman A, Snyder MP, Weissman SM, Zheng D, Skoultchi AI (2012) A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc Natl Acad Sci USA 109:3832–3837. https://doi.org/10.1073/pnas.1121019109
Thesingh CW (1986) Formation sites and distribution of osteoclast progenitor cells during the ontogeny of the mouse. Dev Biol 117:127–134. https://doi.org/10.1016/0012-1606(86)90355-6
Houssaint E (1981) Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ 10:243–252
Zovein AC, Turlo KA, Ponec RM, Lynch MR, Chen KC, Hofmann JJ, Cox TC, Gasson JC, Iruela-Arispe ML (2010) Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood 116:3435–3444. https://doi.org/10.1182/blood-2010-04-279497
Kaufman MH (1992) The atlas of mouse development. Elsevier Academic Press, London
Yamane T, Kunisada T, Yamazaki H, Era T, Nakano T, Hayashi SI (1997) Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood 90:3516–3523. https://doi.org/10.1182/blood.V90.9.3516
Acknowledgements
We thank K Isono for laboratory management and Dr. SI Hayashi for helpful discussions.
Funding
This work was supported by the JSPS KAKENHI (no. 17K09950) to TY and the Takeda Science Foundation to TY.
Author information
Authors and Affiliations
Contributions
TY conceived the experiments; TY and CI designed and performed the experiments; TY and CI analyzed the data; TY, CI, and MHK contributed to conceptualization; HY contributed analysis tools; and TY wrote the paper with input from CI and MHK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Ethics approval and consent to participate
All animal experiments are approved by the institutional review board of Mie University (permission nos. 20–22 and 2021–20). This article does not contain any studies with human participants.
Consent for publication
All authors consent for the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, C., Hikosaka-Kuniishi, M., Yamazaki, H. et al. Multiple cell populations generate macrophage progenitors in the early yolk sac. Cell. Mol. Life Sci. 79, 159 (2022). https://doi.org/10.1007/s00018-022-04203-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04203-7