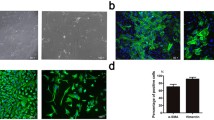
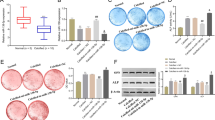
Abstract
Calcific aortic valve disease (CAVD) is a common valve disease characterized by the fibro-calcific remodeling of the aortic valves, which is an actively regulated process involving osteogenic differentiation of valvular interstitial cells (VICs). MicroRNA (miRNA) is an essential regulator in diverse biological processes in cells. The present study aimed to explore the role and mechanism of miR-22 in the osteogenic differentiation of VICs. The expression profile of osteogenesis-related miRNAs was first detected in aortic valve tissue from CAVD patients (n = 33) and healthy controls (n = 12). miR-22 was highly expressed in calcified valve tissues (P < 0.01), and the expression was positively correlated with the expression of OPN (rs = 0.820, P < 0.01) and Runx2 (rs = 0.563, P < 0.01) in VICs isolated from mild or moderately calcified valves. The sustained high expression of miR-22 was also validated in an in-vitro VICs osteogenic model. Adenovirus-mediated gain-of-function and loss-of-function experiments were then performed. Overexpression of miR-22 significantly accelerated the calcification process of VICs, manifested by significant increases in calcium deposition, alkaline phosphate activity, and expression of osteoblastic differentiation markers. Conversely, inhibition of miR-22 significantly negated the calcification process. Subsequently, calcium-binding protein 39 (CAB39) was identified as a target of miR-22. Overexpression of miR-22 significantly reduced the expression of CAB39 in VICs, leading to decreased catalytic activity of the CAB39–LKB1–STRAD complex, which, in turn, exacerbated changes in the AMPK–mTOR signaling pathway, and ultimately accelerated the calcification process. In addition, ROS generation and autophagic activity during VIC calcification were also regulated by miR-22/CAB39 pathway. These results indicate that miR-22 is an important accelerator of the osteogenic differentiation of VICs, and a potential therapeutic target in CAVD.









Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Group ESCSD (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 38(36):2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M (2006) Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011. https://doi.org/10.1016/S0140-6736(06)69208-8
Towler DA (2013) Molecular and cellular aspects of calcific aortic valve disease. Circ Res 113(2):198–208. https://doi.org/10.1161/CIRCRESAHA.113.300155
Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R, Investigators S (2008) Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 359(13):1343–1356. https://doi.org/10.1056/NEJMoa0804602
Mathieu P, Boulanger MC (2014) Basic mechanisms of calcific aortic valve disease. Can J Cardiol 30(9):982–993. https://doi.org/10.1016/j.cjca.2014.03.029
Chen JH, Yip CY, Sone ED, Simmons CA (2009) Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol 174(3):1109–1119. https://doi.org/10.2353/ajpath.2009.080750
Jiang X, Hu C, Arnovitz S, Bugno J, Yu M, Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, Weng H, Strong J, Wang Y, Li Y, Salat J, Li S, Elkahloun AG, Yang Y, Neilly MB, Larson RA, Le Beau MM, Herold T, Bohlander SK, Liu PP, Zhang J, Li Z, He C, Jin J, Hong S, Chen J (2016) miR-22 has a potent anti-tumour role with therapeutic potential in acute myeloid leukaemia. Nat Commun 7:11452. https://doi.org/10.1038/ncomms11452
Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, Xiao Q (2018) miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 137(17):1824–1841. https://doi.org/10.1161/CIRCULATIONAHA.117.027799
Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, Kelm RJ Jr, Reddy AK, Taffet GE, Bradley A, Wehrens XH, Entman ML, Rodriguez A (2012) Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 125(22):2751–2761. https://doi.org/10.1161/CIRCULATIONAHA.111.044354
Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T (2016) Preclinical development of a microRNA-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol 68(14):1557–1571. https://doi.org/10.1016/j.jacc.2016.07.739
Yousry M, Rickenlund A, Petrini J, Jenner J, Liska J, Eriksson P, Franco-Cereceda A, Eriksson MJ, Caidahl K (2015) Aortic valve type and calcification as assessed by transthoracic and transoesophageal echocardiography. Clin Physiol Funct Imaging 35(4):306–313. https://doi.org/10.1111/cpf.12166
Li F, Song R, Ao L, Reece TB, Cleveland JC Jr, Dong N, Fullerton DA, Meng X (2017) ADAMTS5 deficiency in calcified aortic valves is associated with elevated pro-osteogenic activity in valvular interstitial cells. Arterioscler Thromb Vasc Biol 37(7):1339–1351. https://doi.org/10.1161/ATVBAHA.117.309021
Ding X, Yan Y, Zhang C, Xu X, Yang F, Liu Y, Wang G, Qin Y (2021) OCT4 regulated neointimal formation in injured mouse arteries by matrix metalloproteinase 2-mediated smooth muscle cells proliferation and migration. J Cell Physiol 236(7):5421–5431. https://doi.org/10.1002/jcp.30248
Xiao Y, Sun Y, Ma X, Wang C, Zhang L, Wang J, Wang G, Li Z, Tian W, Zhao Z, Jing Q, Zhou J, Jing Z (2020) MicroRNA-22 inhibits the apoptosis of vascular smooth muscle cell by targeting p38MAPKalpha in vascular remodeling of aortic dissection. Mol Ther Nucleic Acids 22:1051–1062. https://doi.org/10.1016/j.omtn.2020.08.018
Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, Bouchareb R, Marchand JT, Nsaibia MJ, Guauque-Olarte S, Pibarot P, Bouchard L, Bosse Y, Mathieu P (2016) Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation 134(23):1848–1862. https://doi.org/10.1161/CIRCULATIONAHA.116.023116
Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E (2007) The role of site accessibility in microRNA target recognition. Nat Genet 39(10):1278–1284. https://doi.org/10.1038/ng2135
Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R (2004) Fast and effective prediction of microRNA/target duplexes. RNA 10(10):1507–1517. https://doi.org/10.1261/rna.5248604
John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS (2004) Human MicroRNA targets. PLoS Biol 2(11):e363. https://doi.org/10.1371/journal.pbio.0020363
Yanagawa B, Lovren F, Pan Y, Garg V, Quan A, Tang G, Singh KK, Shukla PC, Kalra NP, Peterson MD, Verma S (2012) miRNA-141 is a novel regulator of BMP-2-mediated calcification in aortic stenosis. J Thorac Cardiovasc Surg 144(1):256–262. https://doi.org/10.1016/j.jtcvs.2011.10.097
Nigam V, Sievers HH, Jensen BC, Sier HA, Simpson PC, Srivastava D, Mohamed SA (2010) Altered microRNAs in bicuspid aortic valve: a comparison between stenotic and insufficient valves. J Heart Valve Dis 19(4):459–465
Milburn CC, Boudeau J, Deak M, Alessi DR, van Aalten DM (2004) Crystal structure of MO25 alpha in complex with the C terminus of the pseudo kinase STE20-related adaptor. Nat Struct Mol Biol 11(2):193–200. https://doi.org/10.1038/nsmb716
Liu ZL, Li T, Zhu FS, Deng SN, Li XG, He Y (2019) Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis 10(3):227. https://doi.org/10.1038/s41419-019-1373-z
Song R, Fullerton DA, Ao L, Zhao KS, Meng X (2017) An epigenetic regulatory loop controls pro-osteogenic activation by TGF-beta1 or bone morphogenetic protein 2 in human aortic valve interstitial cells. J Biol Chem 292(21):8657–8666. https://doi.org/10.1074/jbc.M117.783308
McCoy CM, Nicholas DQ, Masters KS (2012) Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS One 7(7):e39980. https://doi.org/10.1371/journal.pone.0039980
Cote N, El Husseini D, Pepin A, Guauque-Olarte S, Ducharme V, Bouchard-Cannon P, Audet A, Fournier D, Gaudreault N, Derbali H, McKee MD, Simard C, Despres JP, Pibarot P, Bosse Y, Mathieu P (2012) ATP acts as a survival signal and prevents the mineralization of aortic valve. J Mol Cell Cardiol 52(5):1191–1202. https://doi.org/10.1016/j.yjmcc.2012.02.003
Kamstrup PR, Hung MY, Witztum JL, Tsimikas S, Nordestgaard BG (2017) Oxidized phospholipids and risk of calcific aortic valve disease: the copenhagen general population study. Arterioscler Thromb Vasc Biol 37(8):1570–1578. https://doi.org/10.1161/ATVBAHA.116.308761
Ohukainen P, Syvaranta S, Napankangas J, Rajamaki K, Taskinen P, Peltonen T, Helske-Suihko S, Kovanen PT, Ruskoaho H, Rysa J (2015) MicroRNA-125b and chemokine CCL4 expression are associated with calcific aortic valve disease. Ann Med 47(5):423–429. https://doi.org/10.3109/07853890.2015.1059955
Wang Y, Han D, Zhou T, Zhang J, Liu C, Cao F, Dong N (2020) Melatonin ameliorates aortic valve calcification via the regulation of circular RNA CircRIC3/miR-204-5p/DPP4 signaling in valvular interstitial cells. J Pineal Res 69(2):e12666. https://doi.org/10.1111/jpi.12666
Jia B, Zhang Z, Qiu X, Chu H, Sun X, Zheng X, Zhao J, Li Q (2018) Analysis of the miRNA and mRNA involved in osteogenesis of adipose-derived mesenchymal stem cells. Exp Ther Med 16(2):1111–1120. https://doi.org/10.3892/etm.2018.6303
Liu Z, Li T, Deng S, Fu S, Zhou X, He Y (2018) Radiation induces apoptosis and osteogenic impairment through miR-22-mediated intracellular oxidative stress in bone marrow mesenchymal stem cells. Stem Cells Int 2018:5845402. https://doi.org/10.1155/2018/5845402
Huang S, Wang S, Bian C, Yang Z, Zhou H, Zeng Y, Li H, Han Q, Zhao RC (2012) Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev 21(13):2531–2540. https://doi.org/10.1089/scd.2012.0014
Yin P, Shi Q, Xiao F, Zhao B, Yu W, Wu K, Peng K (2020) Inhibition of miR-22 promotes differentiation of osteoblasts and improves bone formation via the YWHAZ pathway in experimental mice. Arch Med Sci 16(6):1419–1431. https://doi.org/10.5114/aoms.2019.89979
Li L, Tan J, Miao Y, Lei P, Zhang Q (2015) ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol 35(5):615–621. https://doi.org/10.1007/s10571-015-0166-x
Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G (2013) Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol 33(2):e66-74. https://doi.org/10.1161/ATVBAHA.112.300177
Carracedo M, Persson O, Saliba-Gustafsson P, Artiach G, Ehrenborg E, Eriksson P, Franco-Cereceda A, Back M (2019) Upregulated autophagy in calcific aortic valve stenosis confers protection of valvular interstitial cells. Int J Mol Sci 20(6):1486. https://doi.org/10.3390/ijms20061486
Deng XS, Meng X, Venardos N, Song R, Yamanaka K, Fullerton D, Jaggers J (2017) Autophagy negatively regulates pro-osteogenic activity in human aortic valve interstitial cells. J Surg Res 218:285–291. https://doi.org/10.1016/j.jss.2017.05.088
Cui L, Rashdan NA, Zhu D, Milne EM, Ajuh P, Milne G, Helfrich MH, Lim K, Prasad S, Lerman DA, Vesey AT, Dweck MR, Jenkins WS, Newby DE, Farquharson C, Macrae VE (2017) End stage renal disease-induced hypercalcemia may promote aortic valve calcification via Annexin VI enrichment of valve interstitial cell derived-matrix vesicles. J Cell Physiol 232(11):2985–2995. https://doi.org/10.1002/jcp.25935
Bakhshian Nik A, Hutcheson JD, Aikawa E (2017) Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med 4:78. https://doi.org/10.3389/fcvm.2017.00078
Krohn JB, Hutcheson JD, Martinez-Martinez E, Aikawa E (2016) Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol 594(11):2895–2903. https://doi.org/10.1113/JP271338
Mandal K, Raz-Ben Aroush D, Graber ZT, Wu B, Park CY, Fredberg JJ, Guo W, Baumgart T, Janmey PA (2019) Soft hyaluronic gels promote cell spreading, stress fibers, focal adhesion, and membrane tension by phosphoinositide signaling. Not Traction Force ACS Nano 13(1):203–214. https://doi.org/10.1021/acsnano.8b05286
Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K (2012) Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res 93(4):633–644. https://doi.org/10.1093/cvr/cvs007
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14(3):249–256. https://doi.org/10.1038/ncb2441
Hsu CC, Peng D, Cai Z, Lin HK (2021) AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2021.04.006
Van Nostrand JL, Hellberg K, Luo EC, Van Nostrand EL, Dayn A, Yu J, Shokhirev MN, Dayn Y, Yeo GW, Shaw RJ (2020) AMPK regulation of Raptor and TSC2 mediate metformin effects on transcriptional control of anabolism and inflammation. Genes Dev 34(19–20):1330–1344. https://doi.org/10.1101/gad.339895.120
Cai Z, Ding Y, Zhang M, Lu Q, Wu S, Zhu H, Song P, Zou MH (2016) Ablation of adenosine monophosphate-activated protein kinase alpha1 in vascular smooth muscle cells promotes diet-induced atherosclerotic calcification in vivo. Circ Res 119(3):422–433. https://doi.org/10.1161/CIRCRESAHA.116.308301
Dasgupta B, Milbrandt J (2007) Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 104(17):7217–7222. https://doi.org/10.1073/pnas.0610068104
Jiang L, Yan Q, Fang S, Liu M, Li Y, Yuan YF, Li Y, Zhu Y, Qi J, Yang X, Kwong DLW, Guan XY (2017) Calcium-binding protein 39 promotes hepatocellular carcinoma growth and metastasis by activating extracellular signal-regulated kinase signaling pathway. Hepatology 66(5):1529–1545. https://doi.org/10.1002/hep.29312
Acknowledgements
We thank Dr. Chen Wang for discussions and comments on the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81800341, 81800342, and 82000364), National Key Research and Development Program of China (2016YFC1100900), and Shanghai Science and Technology Innovation Action Plan “Science and Technology Support Project in Biomedical Science” (21S11906000).
Author information
Authors and Affiliations
Contributions
GW, ZX, and LZ conceived and designed the study. FY, SL, and YG performed the experiments. FY and XD analyzed the data. FY, and YY collected and analyzed the clinical information. GW and FY wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
The study was approved by the Medical Ethics Committee in Changhai Hospital, and certify that the study was performed in accordance to the principles of the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to enrollment.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, F., Liu, S., Gu, Y. et al. MicroRNA-22 promoted osteogenic differentiation of valvular interstitial cells by inhibiting CAB39 expression during aortic valve calcification. Cell. Mol. Life Sci. 79, 146 (2022). https://doi.org/10.1007/s00018-022-04177-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04177-6