Abstract
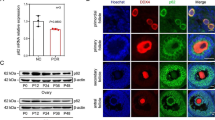
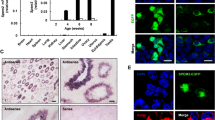
Sertoli cells are essential for spermatogenesis in the testicular seminiferous tubules by forming blood–testis barrier (BTB) and creating a unique microenvironment for spermatogenesis. Many lncRNAs have been reported to participate in spermatogenesis. However, the role of long noncoding RNAs (lncRNAs) in Sertoli cells has rarely been examined. Herein, we found that a high-fat diet (HFD) decreased sperm quality, impaired BTB integrity and resulted in accumulation of saturated fatty acids (SFAs), especially palmitic acid (PA), in mouse testes. PA decreased the expression of tight junction (TJ)-related proteins, increased permeability and decreased transepithelial electrical resistance (TER) in primary Sertoli cells and TM4 cells. Moreover, lncRNA Tug1 was found to be involved in PA-induced BTB disruption by RNA-seq. Tug1 depletion distinctly impaired the TJs of Sertoli cells and overexpression of Tug1 alleviated the disruption of BTB integrity induced by PA. Moreover, Ccl2 was found to be a downstream target of Tug1, and decreased TJ-related protein levels and TER and increased FITC–dextran permeability in vitro. Furthermore, the addition of Ccl2 damaged BTB integrity after overexpression of Tug1 in the presence of PA. Mechanistically, we found that Tug1 could directly bind to EZH2 and regulate H3K27me3 occupancy in the Ccl2 promoter region by RNA immunoprecipitation and chromatin immunoprecipitation assays. Our study revealed an important role of Tug1 in the BTB integrity of Sertoli cells and provided a new view of the role of lncRNAs in male infertility.








Similar content being viewed by others
Availability of data and material
All data generated or analysis during this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Craig JR et al (2017) Obesity, male infertility, and the sperm epigenome. Fertil Steril 107(4):848–859. https://doi.org/10.1016/j.fertnstert.2017.02.115
Eisenberg ML et al (2014) The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 29(2):193–200. https://doi.org/10.1093/humrep/det428
Imterat M et al (2019) Impact of Body Mass Index on female fertility and ART outcomes. Panminerva Med 61(1):58–67. https://doi.org/10.23736/s0031-0808.18.03490-0
Liu Y, Ding Z (2017) Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 154(4):R123-r131. https://doi.org/10.1530/rep-17-0161
Attaman JA et al (2012) Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 27(5):1466–1474. https://doi.org/10.1093/humrep/des065
Salas-Huetos A, Bulló M, Salas-Salvadó J (2017) Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 23(4):371–389. https://doi.org/10.1093/humupd/dmx006
Eslamian G et al (2015) Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil Steril 103(1):190–198. https://doi.org/10.1016/j.fertnstert.2014.10.010
Lu JC et al (2018) Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol 16(1):23. https://doi.org/10.1186/s12958-018-0345-y
Zhao L et al (2020) Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun 11(1):5683. https://doi.org/10.1038/s41467-020-19414-4
Luo D et al (2020) High fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. Life Sci 257:118028. https://doi.org/10.1016/j.lfs.2020.118028
Mruk DD, Cheng CY (2015) The mammalian blood–testis barrier: its biology and regulation. Endocr Rev 36(5):564–591. https://doi.org/10.1210/er.2014-1101
Yang XY et al (2018) Proteomics analysis of testis of rats fed a high-fat diet. Cell Physiol Biochem 47(1):378–389. https://doi.org/10.1159/000489918
Lander ES et al (2001) Initial sequencing and analysis of the human genome. Nature 409(6822):860–921. https://doi.org/10.1038/35057062
Ma L et al (2019) LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res 47(D1):D128-d134. https://doi.org/10.1093/nar/gky960
Zhao XY et al (2018) Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun 9(1):2986. https://doi.org/10.1038/s41467-018-05383-2
de Goede OM et al (2021) Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 184(10):2633-2648.e19. https://doi.org/10.1016/j.cell.2021.03.050
Young TL, Matsuda T, Cepko CL (2005) The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol 15(6):501–512. https://doi.org/10.1016/j.cub.2005.02.027
Sun J et al (2018) LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res 37(1):106. https://doi.org/10.1186/s13046-018-0771-x
Lin YH et al (2018) Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology 67(1):188–203. https://doi.org/10.1002/hep.29462
Long J et al (2016) Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest 126(11):4205–4218. https://doi.org/10.1172/jci87927
Zhang Y et al (2020) LncRNA TUG1 reduces inflammation and enhances insulin sensitivity in white adipose tissue by regulating miR-204/SIRT1 axis in obesity mice. Mol Cell Biochem 475(1–2):171–183. https://doi.org/10.1007/s11010-020-03869-6
Crisóstomo L et al (2020) Diet during early life defines testicular lipid content and sperm quality in adulthood. Am J Physiol Endocrinol Metab 319(6):E1061-e1073. https://doi.org/10.1152/ajpendo.00235.2020
Crisóstomo L et al (2019) A switch from high-fat to normal diet does not restore sperm quality but prevents metabolic syndrome. Reproduction 158(4):377–387. https://doi.org/10.1530/rep-19-0259
Lewandowski JP et al (2020) The Tug1 lncRNA locus is essential for male fertility. Genome Biol 21(1):237. https://doi.org/10.1186/s13059-020-02081-5
Cai H et al (2015) The long noncoding RNA TUG1 regulates blood–tumor barrier permeability by targeting miR-144. Oncotarget 6(23):19759–19779. https://doi.org/10.18632/oncotarget.4331
Gril B et al (2018) Reactive astrocytic S1P3 signaling modulates the blood–tumor barrier in brain metastases. Nat Commun 9(1):2705. https://doi.org/10.1038/s41467-018-05030-w
Li J et al (2020) High fat diet induced obesity model using four strains of mice: kunming, C57BL/6, BALB/c and ICR. Exp Anim 69(3):326–335. https://doi.org/10.1538/expanim.19-0148
Chen Y et al (2018) Microcystin-leucine-arginine causes blood–testis barrier disruption and degradation of occludin mediated by matrix metalloproteinase-8. Cell Mol Life Sci 75(6):1117–1132. https://doi.org/10.1007/s00018-017-2687-6
Xu D et al (2020) Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J Pineal Res 69(4):e12690. https://doi.org/10.1111/jpi.12690
Hu X et al (2018) Effects of saturated palmitic acid and omega-3 polyunsaturated fatty acids on Sertoli cell apoptosis. Syst Biol Reprod Med 64(5):368–380. https://doi.org/10.1080/19396368.2018.1471554
Stamatovic SM et al (2006) Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem 281(13):8379–8388. https://doi.org/10.1074/jbc.M513122200
Wang S et al (2020) Omega-3 polyunsaturated fatty acids alleviate hydrogen sulfide-induced blood–testis barrier disruption in the testes of adult mice. Reprod Toxicol 98:233–241. https://doi.org/10.1016/j.reprotox.2020.10.007
Sun X et al (2017) AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ 24(5):819–831. https://doi.org/10.1038/cdd.2017.14
Tarulli GA et al (2008) Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction 135(6):867–877. https://doi.org/10.1530/rep-07-0572
Ma B et al (2019) Luteolin ameliorates testis injury and blood–testis barrier disruption through the Nrf2 signaling pathway and by upregulating Cx43. Mol Nutr Food Res 63(10):e1800843. https://doi.org/10.1002/mnfr.201800843
Joshi M, Rajender S (2020) Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod Biol Endocrinol 18(1):103. https://doi.org/10.1186/s12958-020-00660-6
Pérez CV et al (2014) IL17A impairs blood–testis barrier integrity and induces testicular inflammation. Cell Tissue Res 358(3):885–898. https://doi.org/10.1007/s00441-014-1995-5
Huang MD et al (2015) Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer 14:165. https://doi.org/10.1186/s12943-015-0431-0
Duan R, Du W, Guo W (2020) EZH2: a novel target for cancer treatment. J Hematol Oncol 13(1):104. https://doi.org/10.1186/s13045-020-00937-8
Fan Y et al (2015) Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood–testis barrier. PLoS ONE 10(4):e0120775. https://doi.org/10.1371/journal.pone.0120775
Cheng CY, Mruk DD (2012) The blood–testis barrier and its implications for male contraception. Pharmacol Rev 64(1):16–64. https://doi.org/10.1124/pr.110.002790
Keaney J, Campbell M (2015) The dynamic blood–brain barrier. FEBS J 282(21):4067–4079. https://doi.org/10.1111/febs.13412
Song L, Ge S, Pachter JS (2007) Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 109(4):1515–1523. https://doi.org/10.1182/blood-2006-07-034009
Echeverry S et al (2011) Peripheral nerve injury alters blood–spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci 31(30):10819–10828. https://doi.org/10.1523/jneurosci.1642-11.2011
Rangasamy S et al (2014) Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood–retinal barrier in diabetic retinopathy. PLoS ONE 9(10):e108508. https://doi.org/10.1371/journal.pone.0108508
Xu Y et al (2017) The lncRNA TUG1 modulates proliferation in trophoblast cells via epigenetic suppression of RND3. Cell Death Dis 8(10):e3104. https://doi.org/10.1038/cddis.2017.503
Niu Y et al (2017) Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer 16(1):5. https://doi.org/10.1186/s12943-016-0575-6
Laugesen A, Højfeldt JW, Helin K (2019) Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell 74(1):8–18. https://doi.org/10.1016/j.molcel.2019.03.011
Smith LB, Walker WH (2014) The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. https://doi.org/10.1016/j.semcdb.2014.02.012
Acknowledgements
We thank the other members of Dr. Yao’s laboratory for their discussion and help.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2018YFC1004700), the National Natural Science Foundation of China (grant no. 81971373, 82001618, 81901547), the Natural Science Foundation of Jiangsu Province (BK20190252), and the 333 High-level Personnel Training Project of Jiangsu Province (grant no. BRA2019109).
Author information
Authors and Affiliations
Contributions
S.W., Z.Q., C.L., L.Z. and K.L. carried out the experiments. R.M., X.G. and J.J. were involved in planning and supervising the work. M.X., L.O. and Y.Z. helped with the animal experiments. S.C., Y.C. and Y.Y. analyzed the data and designed the figures. S.W and Z.Q. wrote the manuscript with support from J.M. and B.Y.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Ethics Committee of Nanjing Jinling Hospital.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4142_MOESM1_ESM.tif
Supplementary file1 Fig. S1 HFD impaired BTB integrity (a) The simple schematic diagram of animal experiment design in this study. (b-c) The immunofluorescence staining of BTB-related genes in the CD and HFD group. Scale bar = 50 μm. * p < 0.05 (TIF 2902 KB)
18_2022_4142_MOESM3_ESM.tif
Supplementary file3 Fig. S3 FSH supplementation did not rescue the sperm quality and BTB integrity in HFD group (n>5) (a) FSH level in control group, HFD group with or without FSH supplementation. (b-c) Sperm concentration and motility of control group, HFD group with or without FSH supplementation. (d) FITC tracing assay of control group, HFD group with or without FSH supplementation. FITC green fluorescence was seen in the lumen of HFD group and HFD group with FSH supplementation. * p < 0.05 (TIF 1011 KB)
18_2022_4142_MOESM4_ESM.tif
Supplementary file4 Fig. S4 GO and KEGG analysis of different expression genes of the testes of tug1-/- and wild type (WT) mice (a) GO terms of different expression genes of the testes of tug1-/- and WT mice. (b) The top 20 KEGG pathways of differentially expression genes. The right panel showed genes involved in tight junction pathway. (TIF 4240 KB)
Rights and permissions
About this article
Cite this article
Wang, S., Qian, Z., Ge, X. et al. LncRNA Tug1 maintains blood–testis barrier integrity by modulating Ccl2 expression in high-fat diet mice. Cell. Mol. Life Sci. 79, 114 (2022). https://doi.org/10.1007/s00018-022-04142-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04142-3