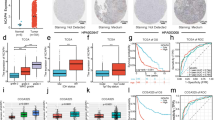
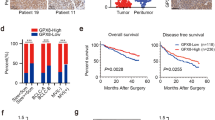
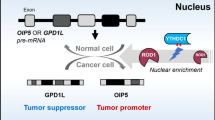
Abstract
Oxaliplatin is the first-line regime for advanced gastric cancer treatment, while its resistance is a major problem that leads to the failure of clinical treatments. Tumor cell heterogeneity has been considered as one of the main causes for drug resistance in cancer. In this study, the mechanism of oxaliplatin resistance was investigated through in vitro human gastric cancer organoids and gastric cancer oxaliplatin-resistant cell lines and in vivo subcutaneous tumorigenicity experiments. The in vitro and in vivo results indicated that CD133+ stem cell-like cells are the main subpopulation and PARP1 is the central gene mediating oxaliplatin resistance in gastric cancer. It was found that PARP1 can effectively repair DNA damage caused by oxaliplatin by means of mediating the opening of base excision repair pathway, leading to the occurrence of drug resistance. The CD133+ stem cells also exhibited upregulated expression of N6-methyladenosine (m6A) mRNA and its writer METTL3 as showed by immunoprecipitation followed by sequencing and transcriptome analysis. METTTL3 enhances the stability of PARP1 by recruiting YTHDF1 to target the 3′-untranslated Region (3′-UTR) of PARP1 mRNA. The CD133+ tumor stem cells can regulate the stability and expression of m6A to PARP1 through METTL3, and thus exerting the PARP1-mediated DNA damage repair ability. Therefore, our study demonstrated that m6A Methyltransferase METTL3 facilitates oxaliplatin resistance in CD133+ gastric cancer stem cells by Promoting PARP1 mRNA stability which increases base excision repair pathway activity.









Similar content being viewed by others
Availability of data and materials
The tumor data of BALB/C NUDE mice have been deposited in the NCBI BioProject database (www.ncbi.nlm.nih.gov/bioproject) under BioProject accession no. PRJNA669425, PRJNA669419. The RNA-Seq data in gastric cancer in the TCGA database was downloaded from https://cptac-data-portal.georgetown.edu/study-summary/S025. Obtain single-cell sequencing data of early gastric cancer tissue from the GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE134520). All relevant data could be obtained from the corresponding author.
References
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I (2020) Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69(9):1564–1571
Zong L, Abe M, Seto Y, Ji J (2016) The challenge of screening for early gastric cancer in China. Lancet (London, England) 388(10060):2606
Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW et al (2019) Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol Off J Eur Soc Med Oncol 30(2):250–258
Harada K, Sakamoto N, Ukai S, Yamamoto Y, Pham QT, Taniyama D, et al. Establishment of oxaliplatin-resistant gastric cancer organoids: importance of myoferlin in the acquisition of oxaliplatin resistance. Gastric Cancer 2021.
Wang VM, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M et al (2019) CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol 21(11):1425–1435
Plaks V, Kong N, Werb Z (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16(3):225–238
Xu M, Gong A, Yang H, George SK, Jiao Z, Huang H et al (2015) Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44(+)/Musashi-1(+) gastric cancer stem cells. Cancer Lett 369(1):124–133
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R et al (2009) Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells (Dayton, Ohio) 27(5):1006–1020
Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M et al (2013) A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells in S/G2/M phases. Clin Cancer Res Off J Am Assoc Cancer Res 19(23):6495–6505
Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova II (2018) Therapy resistance mediated by cancer stem cells. Semin Cancer Biol 53:156–167
Bai X, Ni J, Beretov J, Graham P, Li Y (2018) Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev 69:152–163
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y et al (2017) The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer 16(1):52
Huang L, Cai J, Guo H, Gu J, Tong Y, Qiu B et al (2019) ID3 promotes stem cell features and predicts chemotherapeutic response of intrahepatic cholangiocarcinoma. Hepatol (Baltimore, MD) 69(5):1995–2012
Lucena-Cacace A, Otero-Albiol D, Jiménez-García MP, Muñoz-Galvan S, Carnero A (2018) NAMPT is a potent oncogene in colon cancer progression that modulates cancer stem cell properties and resistance to therapy through Sirt1 and PARP. Clin Cancer Res Off J Am Assoc Cancer Res 24(5):1202–1215
Ni SJ, Zhao LQ, Wang XF, Wu ZH, Hua RX, Wan CH et al (2018) CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-κB-miR-21 pathways. J Hematol Oncol 11(1):17
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760
Vitale I, Manic G, De Maria R, Kroemer G, Galluzzi L (2017) DNA damage in stem cells. Mol Cell 66(3):306–319
Chelmicki T, Roger E, Teissandier A, Dura M, Bonneville L, Rucli S et al (2021) m(6)A RNA methylation regulates the fate of endogenous retroviruses. Nature 591(7849):312–316
Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V et al (2018) Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene 37(4):522–533
Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L et al (2018) METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell 22(2):191-205.e199
Bai Y, Yang C, Wu R, Huang L, Song S, Li W et al (2019) YTHDF1 regulates tumorigenicity and cancer stem cell-like activity in human colorectal carcinoma. Front Oncol 9:332
Zhang C, Huang S, Zhuang H, Ruan S, Zhou Z, Huang K et al (2020) YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene 39(23):4507–4518
Dixit D, Prager BC, Gimple RC, Poh HX, Wang Y, Wu Q et al (2021) The RNA m6A Reader YTHDF2 maintains oncogene expression and is a targetable dependency in glioblastoma stem cells. Cancer Discov 11(2):480–499
Jin D, Guo J, Wu Y, Du J, Yang L, Wang X et al (2019) m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol 12(1):135
Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X et al (2020) RNA m6A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J 39(12):e103181
Liu X, Gonzalez G, Dai X, Miao W, Yuan J, Huang M et al (2020) Adenylate kinase 4 modulates the resistance of breast cancer cells to tamoxifen through an m6A-based epitranscriptomic mechanism. Mol Ther 28(12):2593–2604
Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grützmann K et al (2019) Human gastric cancer modelling using organoids. Gut 68(2):207–217
Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L et al (2019) RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer 18(1):46
Ruiz EJ, Diefenbacher ME, Nelson JK, Sancho R, Pucci F, Chakraborty A et al (2019) LUBAC determines chemotherapy resistance in squamous cell lung cancer. J Exp Med 216(2):450–465
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN et al (2018) Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173(2):338-354.e315
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf 12:323
Snel B, Lehmann G, Bork P, Huynen MA (2000) STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res 28(18):3442–3444
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M et al (2015) Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161(5):1202–1214
Zhang Y, Zhang Y, Hu J, Zhang J, Guo F, Zhou M et al (2020) scTPA: a web tool for single-cell transcriptome analysis of pathway activation signatures. Bioinf (Oxf, Engl) 36(14):4217–4219
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (Lond, Engl) 393(10184):1948–1957
Liu T, Zhang X, Du L, Wang Y, Liu X, Tian H et al (2019) Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer 18(1):43
Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14(10):611–629
Martinez-Balibrea E, Martínez-Cardús A, Ginés A, Ruizorras V, Moutinho C, Layos L et al (2015) Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Ther 14(8):1767–1776
Park SY, Lee CJ, Choi JH, Kim JH, Kim JW, Kim JY et al (2019) The JAK2/STAT3/CCND2 axis promotes colorectal cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res CR 38(1):399
Noordermeer SM, van Attikum H (2019) PARP inhibitor resistance: a tug-of-war in BRCA-mutated cells. Trends Cell Biol 29(10):820–834
Bell NAW, Haynes PJ, Brunner K, de Oliveira TM, Flocco MM, Hoogenboom BW et al (2021) Single-molecule measurements reveal that PARP1 condenses DNA by loop stabilization. Sci Adv 7(33):3641
Singh MP, Cho HJ, Kim JT, Baek KE, Lee HG, Kang SC (2019) Morin hydrate reverses cisplatin resistance by impairing PARP1/HMGB1-dependent autophagy in hepatocellular carcinoma. Cancers 11(7)
Hu K, Wu W, Li Y, Lin L, Chen D, Yan H et al (2019) Poly(ADP-ribosyl)ation of BRD7 by PARP1 confers resistance to DNA-damaging chemotherapeutic agents. EMBO Rep 20(5)
Avitabile M, Lasorsa VA, Cantalupo S, Cardinale A, Cimmino F, Montella A et al (2020) Association of PARP1 polymorphisms with response to chemotherapy in patients with high-risk neuroblastoma. J Cell Mol Med 24(7):4072–4081
Slyskova J, Sabatella M, Ribeiro-Silva C, Stok C, Theil AF, Vermeulen W et al (2018) Base and nucleotide excision repair facilitate resolution of platinum drugs-induced transcription blockage. Nucleic Acids Res 46(18):9537–9549
Ronson GE, Piberger AL, Higgs MR, Olsen AL, Stewart GS, McHugh PJ et al (2018) PARP1 and PARP2 stabilise replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat Commun 9(1):746
Reynolds P, Cooper S, Lomax M, O’Neill P (2015) Disruption of PARP1 function inhibits base excision repair of a sub-set of DNA lesions. Nucleic Acids Res 43(8):4028–4038
Lan Q, Liu PY, Haase J, Bell JL, Hüttelmaier S, Liu T (2019) The critical role of RNA m(6)A methylation in cancer. Can Res 79(7):1285–1292
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C et al (2019) Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun 10(1):1858
Hao L, Wang JM, Liu BQ, Yan J, Li C, Jiang JY et al (2021) m6A-YTHDF1-mediated TRIM29 upregulation facilitates the stem cell-like phenotype of cisplatin-resistant ovarian cancer cells. Biochim Et Biophys Acta Mol Cell Res 1868(1):118878
Wang H, Meng Q, Ma B (2021) Characterization of the prognostic m6A-Related lncRNA signature in gastric cancer. Front Oncol 11:630260
Garbo S, Zwergel C, Battistelli C (2021) m6A RNA methylation and beyond—the epigenetic machinery and potential treatment options. Drug Discov Today 26(11):2559–2574
Acknowledgements
The authors would like to thank Professor Antonio Tedeschi (Adult Stem Cell Laboratory, The Francis Crick Institute) for valuable comments on this article.
Funding
This study was supported by the National Natural Science Foundation of China (grant No. 82073148), the Sanming Project of Medicine in Shenzhen (SZSM201911010), the Shenzhen Key Medical Discipline Construction Fund (SZXK016), the Shenzhen Sustainable Project (KCXFZ202002011010593), Shenzhen-Hong Kong-Macau Technology Research Programme (Type C) (Grant No. SGDX2020110309260100) and the Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006).
Author information
Authors and Affiliations
Contributions
All authors participated in the writing of the manuscript. AB, YH, CZ, HL and RLR designed the study. HL, CW and LL worked on the methodologies. HL, CW, LL, IE, WL, QS and ZZ performed data collection (providing animals, collection of patients samples, providing facilities, etc.). HL, CW, LL, ER conducted data analysis and interpretation (for example, statistical analysis, biostatistics, computational analysis). HL, CW, WC, ZH, LY were responsible for cultivation and development of organoids. LY, JMO and RLR supervised the study and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Ethical Review [2018] No. 087). And the clinical research and animal experiment ethics committee of the First Affiliated Hospital of Sun Yat-sen University (Ethical Review [2017] No. 208).
Consent for publication
We understand that journals may be available in both print and on the internet, and will be available to a broader audience through marketing channels and other third parties. I understand that readers may include not only medical professionals and scholarly researchers but also journalists and general members of the public.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4129_MOESM1_ESM.pdf
Figure S1. Verifying the drug resistance level in GC resistant strains. (A) Representative images of colony formation of SNU719, MKN74 and AGS resistant strains and their corresponding wild-type cell lines in the same dose of oxaliplatin. (B) Comparison of the number of colonies in (A). (C-E) Indicate the comparison of cell viability between AGS, SNU719 and MNK74 Oxaliplatin resistance strains and wild-type cell strains under the effects of oxaliplatin respectively. OXA: Oxaliplatin. OLP: Olaparib. CON: Control group. AGSRE: AGS oxaliplatin resistance. SNU719RE: SNU719 oxaliplatin resistance. MKN74RE: MKN74 oxaliplatin resistance. * p < 0.05, ** p < 0.01, *** p < 0.001 (PDF 1686 KB)
18_2022_4129_MOESM2_ESM.pdf
Figure S2. Oxaliplatin-resistant human gastric cancer cell lines enrich for CD133+ cells. (A) Representative image of the comparison of the spheroidizing ability of SNU719, MKN74, AGS-resistant strains and their wild-type cell lines. (B) Proportion of spheroids in (A). (C-E) RT-qPCR was used to compare the mRNA expression levels of SNU719, MKN74 and AGS resistant strains and their wild-type cell lines LGR5, CD133, and CD44 respectively. (F) Flow cytometric analysis and comparison of CD133+ cells in SNU719, AGS and MKN74 resistant strains and their corresponding wild-type cell lines. Isotype is a control without antibody. The abscissa represents CD133, and the ordinate represents cells. * p < 0.05, ** p < 0.01, *** p < 0.001. (PDF 1870 KB)
18_2022_4129_MOESM3_ESM.pdf
Figure S3. CD133+ cell show highly stem-like properties. (A) A representative image of the spheroidization experiment based on the cells processed in flow cytometry. (B) The proportion of spheroids in (A). (C, D) RT-qPCR was performed on the cells sorted in H and their mRNA differences were analyzed. OXA: Oxaliplatin. OLP: Olaparib. CON: Control group. AGSRE: AGS oxaliplatin resistance. SNU719RE: SNU719 oxaliplatin resistance. MKN74RE: MKN74 oxaliplatin resistance. * p < 0.05, ** p < 0.01, *** p < 0.001 (PDF 4920 KB)
18_2022_4129_MOESM4_ESM.pdf
Figure S4. CD133 and PARP1 are elevated in CSCs. (A) Volcano plot for the statistical significance (y-axis, log2 transformation) versus the fold change of gene expression (x-axis, log2 transformation) between populations with high and low stemness indices determined by RNA-seq (n = 3). FC, fold change; padj, false-discovery-rate adjusted p value. The red dots denote significantly differentially expressed genes with padj < 0.05 of low stemness. The green dots denote significantly differentially expressed genes with padj < 0.05 of high stemness. (B-E) Stemness index is highly enriched in JAK STAT signaling pathway, MAPK Signaling pathway, TGF BETA signaling pathway, WNT Signaling pathway (PDF 3013 KB)
18_2022_4129_MOESM5_ESM.pdf
Figure S5. CD133+ cells are a subgroup of cells capable of self-renewal and DNA repair. (A, B) Nonlinear dimension reduction analysis of pathway activity score using t-SNE and UMAP method. (C) The heatmap of cell-type-specific activation pathways. (D) The ratio of other groups of CD133+ cells. (E) The first eight sites of C6 and C5 enrichment pathways (PDF 3788 KB)
18_2022_4129_MOESM6_ESM.pdf
Figure S6. The expression of PARP1 and CD133 in PT2, PT4, CD133+, CD133-, pLKO and SH-mettl3 were verified by WB. (A) comparison of PARP1 expression between PT2 and PT4. (B) comparison of expression of CD133+ and CD133-organoidPARP1. (C) CD133+ and CD133- PARP1 expression in subcutaneous tumors. (D) comparison of PARP1 expression in pLKO and SH-MettL3 organs and subcutaneous tumors (PDF 973 KB)
18_2022_4129_MOESM7_ESM.pdf
Figure S7. PARP1 and METTL3 were elevated in CSCs and oxaliplatin resistance cells. (A) RT-qPCR was used to compare the expression of PARP1 mRNA in SNU719, MKN74 and AGS-resistant CD133+ and CD133- cells. (B) RT-qPCR was used to compare PARP1 mRNA expression in SNU719, MKN74 and AGS resistant strains and their wild-type cell lines. (C) RT-qPCR was used to compare the expression of METTL3 mRNA in SNU719, MKN74 and AGS-resistant CD133+ and CD133- cells. (D) RT-qPCR was used to compare METTL3 mRNA expression in SNU719, MKN74 and AGS resistant strains and their wild-type cell lines. (E) By comparing the expression levels of PARP1 of SNU719, MKN74 and AGS resistant strains and their wild-type cell lines. (F) By comparing the expression levels of METTL3 of SNU719, MKN74 and AGS resistant strains and their wild-type cell lines. AGSRE: AGS oxaliplatin resistance. SNU719RE: SNU719 oxaliplatin resistance. MKN74RE: MKN74 oxaliplatin resistance. * p < 0.05, ** p < 0.01, *** p < 0.001. (PDF 1137 KB)
18_2022_4129_MOESM8_ESM.pdf
Figure S8. PARP1 knockdown and overexpression were verified by WB. (A) Comparing the expression levels of PARP1 of PT1 pLKO, PT1 sh1, PT1 sh2, PT1 sh3. (B) Comparing the expression levels of PARP1 of PT2 pLKO, PT2 sh1, PT2 sh2, PT2 sh3. (C) Comparing the expression levels of PARP1 of PT3 CON, PT3 PARP1, PT4 CON, PT4 PARP1 (PDF 1305 KB)
18_2022_4129_MOESM9_ESM.pdf
Figure S9. CD133+ cells were enriched in tumor stem cells and DNA damage repair related pathways and RNA modification. (A, B) CD133+ CSCs were enriched in tumor stem cells and DNA damage repair related pathways and RNA modification. (C, D) WB analysis of METTL3 and YTHDF1 in successfully transfected stable strains. (E, F) WB analysis of PT3 and PT4 organoids following METTL3 knockdown and PARP1 overexpression, PARP1 knockdown and METTL3 overexpression, METTL3 overexpression, PARP1 overexpression, METTL3 overexpression and PARP1 overexpression (PDF 631 KB)
18_2022_4129_MOESM10_ESM.pdf
Figure S10. m6A positive and negative controls and Dual Luciferase plasmid system validation. (A, B, C) MerIP-QPCR positive and negative controls indicated that the experimental results were reliable. (D) RNA decay rate positive and negative controls indicated that the experimental results were reliable. (E, F) RNA decay rate followed by Dual luciferase plasmid system assay demonstrated the PARP1 mRNA half-lives upon the METTL3 knockdown and YTHDF1 knockdown. Data were detected at indicated timepoint with actinomycin D (Act D, 5 μg/mL) treatment (PDF 488 KB)
Rights and permissions
About this article
Cite this article
Li, H., Wang, C., Lan, L. et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell. Mol. Life Sci. 79, 135 (2022). https://doi.org/10.1007/s00018-022-04129-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04129-0