Abstract
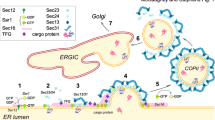
Secretion and quality control of large extracellular matrix proteins remain poorly understood and debated, particularly transport intermediates delivering folded proteins from the ER to Golgi and misfolded ones to lysosomes. Discrepancies between different studies are related to utilization of exogenous cargo, off-target effects of experimental conditions and cell manipulation, and identification of transport intermediates without tracing their origin and destination. To address these issues, here we imaged secretory and degradative trafficking of type I procollagen in live MC3T3 osteoblasts by replacing a region encoding N-propeptide in endogenous Col1a2 gDNA with GFP cDNA. We selected clones that produced the resulting fluorescent procollagen yet had normal expression of key osteoblast and ER/cell stress genes, normal procollagen folding, and normal deposition and mineralization of extracellular matrix. Live-cell imaging of these clones revealed ARF1-dependent transport intermediates, which had no COPII coat and delivered procollagen from ER exit sites (ERESs) to Golgi without stopping at ER–Golgi intermediate compartment (ERGIC). It also confirmed ERES microautophagy, i.e., lysosomes engulfing ERESs containing misfolded procollagen. Beyond validating these trafficking models for endogenous procollagen, we uncovered a probable cause of noncanonical cell stress response to procollagen misfolding. Recognized and retained only at ERESs, misfolded procollagen does not directly activate the canonical UPR, yet it disrupts the ER lumen by blocking normal secretory export from the ER.







Similar content being viewed by others
Data availability
All cell lines, plasmids, and data generated in this study will be deposited to public repositories or freely provided upon request after acceptance of the paper for publication.
References
Prockop DJ, Kivirikko KI (1995) Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64:403–434. https://doi.org/10.1146/annurev.bi.64.070195.002155
Canty EG, Kadler KE (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118:1341–1353. https://doi.org/10.1242/jcs.01731
Ishikawa Y, Bachinger HP (2013) A molecular ensemble in the rER for procollagen maturation. Biochim Biophys Acta 1833:2479–2491. https://doi.org/10.1016/j.bbamcr.2013.04.008
Koide T, Nagata K (2005) Collagen biosynthesis. In: Brinckmann J, Notbohm H, Muller PK (eds) Collagen: primer in structure, processing and assembly. Topics in current chemistry. Springer, Heidelberg, pp 85–114. https://doi.org/10.1007/b103820
Makareeva E, Aviles NA, Leikin S (2011) Chaperoning osteogenesis: new protein-folding disease paradigms. Trends Cell Biol 21:168–176. https://doi.org/10.1016/j.tcb.2010.11.007
Barile FA, Guzowski DE, Ripley C, Siddiqi ZA, Bienkowski RS (1990) Ammonium chloride inhibits basal degradation of newly synthesized collagen in human fetal lung fibroblasts. Arch Biochem Biophys 276:125–131. https://doi.org/10.1016/0003-9861(90)90018-t
Berg RA, Schwartz ML, Crystal RG (1980) Regulation of the production of secretory proteins: intracellular degradation of newly synthesized “defective” collagen. Proc Natl Acad Sci USA 77:4746–4750. https://doi.org/10.1073/pnas.77.8.4746
Bateman JF, Boot-Handford RP, Lamande SR (2009) Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet 10:173–183. https://doi.org/10.1038/nrg2520
Forlino A, Marini JC (2016) Osteogenesis imperfecta. Lancet 387:1657–1671. https://doi.org/10.1016/S0140-6736(15)00728-X
Omari S, Makareeva E, Leikin S (2021) Procollagen trafficking and its implications in OI. In: Ruggiero F (ed) Collagen superfamily and collagenopathies. Springer, Heidelberg (In press)
Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q, Zou S (2019) Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res 7:28. https://doi.org/10.1038/s41413-019-0058-7
Venditti R, Wilson C, De Matteis MA (2014) Exiting the ER: what we know and what we don’t. Trends Cell Biol 24:9–18. https://doi.org/10.1016/j.tcb.2013.08.005
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R (2011) COPII and the regulation of protein sorting in mammals. Nat Cell Biol 14:20–28. https://doi.org/10.1038/ncb2390
Gorur A, Yuan L, Kenny SJ, Baba S, Xu K, Schekman R (2017) COPII-coated membranes function as transport carriers of intracellular procollagen I. J Cell Biol 216:1745–1759. https://doi.org/10.1083/jcb.201702135
Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M (2012) Ubiquitin-dependent regulation of COPII coat size and function. Nature 482:495–500. https://doi.org/10.1038/nature10822
Yuan L, Kenny SJ, Hemmati J, Xu K, Schekman R (2018) TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers. Proc Natl Acad Sci USA 115:E12255–E12264. https://doi.org/10.1073/pnas.1814810115
Nogueira C, Erlmann P, Villeneuve J, Santos AJ, Martinez-Alonso E, Martinez-Menarguez JA, Malhotra V (2014) SLY1 and syntaxin 18 specify a distinct pathway for procollagen VII export from the endoplasmic reticulum. Elife 3:e02784. https://doi.org/10.7554/eLife.02784
McCaughey J, Stevenson NL, Cross S, Stephens DJ (2019) ER-to-Golgi trafficking of procollagen in the absence of large carriers. J Cell Biol 218:929–948. https://doi.org/10.1083/jcb.201806035
Omari S, Makareeva E, Gorrell L, Jarnik M, Lippincott-Schwartz J, Leikin S (2020) Mechanisms of procollagen and HSP47 sorting during ER-to-Golgi trafficking. Matrix Biol 93:79–94. https://doi.org/10.1016/j.matbio.2020.06.002
Fitzgerald J, Lamande SR, Bateman JF (1999) Proteasomal degradation of unassembled mutant type I collagen pro-alpha1(I) chains. J Biol Chem 274:27392–27398. https://doi.org/10.1074/jbc.274.39.27392
Ishida Y, Yamamoto A, Kitamura A, Lamande SR, Yoshimori T, Bateman JF, Kubota H, Nagata K (2009) Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell 20:2744–2754. https://doi.org/10.1091/mbc.E08-11-1092
Lamande SR, Chessler SD, Golub SB, Byers PH, Chan D, Cole WG, Sillence DO, Bateman JF (1995) Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the pro alpha 1 (I) chain carboxyl-terminal propeptide which impair subunit assembly. J Biol Chem 270:8642–8649. https://doi.org/10.1074/jbc.270.15.8642
Doan ND, Hosseini AS, Bikovtseva AA, Huang MS, DiChiara AS, Papa LJ 3rd, Koller A, Shoulders MD (2020) Elucidation of proteostasis defects caused by osteogenesis imperfecta mutations in the collagen-alpha2(I) C-propeptide domain. J Biol Chem 295:9959–9973. https://doi.org/10.1074/jbc.RA120.014071
Ishida Y, Nagata K (2009) Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy 5:1217–1219. https://doi.org/10.4161/auto.5.8.10168
Forrester A, De Leonibus C, Grumati P, Fasana E, Piemontese M, Staiano L et al (2019) A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. EMBO J. https://doi.org/10.15252/embj.201899847
Fregno I, Fasana E, Solda T, Galli C, Molinari M (2021) N-glycan processing selects ERAD-resistant misfolded proteins for ER-to-lysosome-associated degradation. EMBO J. https://doi.org/10.15252/embj.2020107240
Reggio A, Buonomo V, Berkane R, Bhaskara RM, Tellechea M, Peluso I et al (2021) Role of FAM134 paralogues in endoplasmic reticulum remodeling, ER-phagy, and Collagen quality control. EMBO Rep. https://doi.org/10.15252/embr.202052289
Omari S, Makareeva E, Roberts-Pilgrim A, Mirigian L, Jarnik M, Ott C, Lippincott-Schwartz J, Leikin S (2018) Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci USA 115:E10099–E10108. https://doi.org/10.1073/pnas.1814552115
Fregno I, Molinari M (2019) Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol 54:153–163. https://doi.org/10.1080/10409238.2019.1610351
Lippincott-Schwartz J, Roberts TH, Hirschberg K (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol 16:557–589. https://doi.org/10.1146/annurev.cellbio.16.1.557
Song F, Stieger K (2017) Optimizing the DNA donor template for homology-directed repair of double-strand breaks. Mol Ther Nucl Acids 7:53–60. https://doi.org/10.1016/j.omtn.2017.02.006
Makareeva E, Mertz EL, Kuznetsova NV, Sutter MB, DeRidder AM, Cabral WA, Barnes AM, McBride DJ, Marini JC, Leikin S (2008) Structural heterogeneity of type I collagen triple helix and its role in osteogenesis imperfecta. J Biol Chem 283:4787–4798. https://doi.org/10.1074/jbc.M705773200
Daley E, Streeten EA, Sorkin JD, Kuznetsova N, Shapses SA, Carleton SM, Shuldiner AR, Marini JC, Phillips CL, Goldstein SA, Leikin S, McBride DJ Jr (2010) Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. J Bone Miner Res 25:247–261. https://doi.org/10.1359/jbmr.090720
American Type Culture Collection Standards Development Organization Workgroup ASN (2010) Cell line misidentification: the beginning of the end. Nat Rev Cancer 10:441–8. https://doi.org/10.1038/nrc2852
Tanabe H, Takada Y, Minegishi D, Kurematsu M, Masui T, Mizusawa H (1999) Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24. Tiss Cult Res Commun 18:329–338. https://doi.org/10.11418/jtca1981.18.4_329
Mirigian LS, Makareeva E, Mertz EL, Omari S, Roberts-Pilgrim AM, Oestreich AK, Phillips CL, Leikin S (2016) Osteoblast malfunction caused by cell stress response to procollagen misfolding in alpha2(I)-G610C mouse model of osteogenesis imperfecta. J Bone Miner Res 31:1608–1616. https://doi.org/10.1002/jbmr.2824
Peterkofsky B (1991) Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr 54:1135s-s1140. https://doi.org/10.1093/ajcn/54.6.1135s
Stephens DJ, Allan VJ (2003) Light microscopy techniques for live cell imaging. Science 300:82–86. https://doi.org/10.1126/science.1082160
Lippincott-Schwartz J (2011) Emerging in vivo analyses of cell function using fluorescence imaging (*). Annu Rev Biochem 80:327–332. https://doi.org/10.1146/annurev-biochem-121010-125553
Lemon WC, McDole K (2020) Live-cell imaging in the era of too many microscopes. Curr Opin Cell Biol 66:34–42. https://doi.org/10.1016/j.ceb.2020.04.008
Specht EA, Braselmann E, Palmer AE (2017) A critical and comparative review of fluorescent tools for live-cell imaging. Annu Rev Physiol 79:93–117. https://doi.org/10.1146/annurev-physiol-022516-034055
Calverley BC, Kadler KE, Pickard A (2020) Dynamic high-sensitivity quantitation of procollagen-I by endogenous CRISPR-cas9 nanoluciferase tagging. Cells 9:2070. https://doi.org/10.3390/cells9092070
Pickard A, Adamson A, Lu Y, Chang J, Garva R, Hodson N, Kadler KE (2018) Collagen assembly and turnover imaged with a CRISPR-Cas9 engineered dendra2 tag. bioRxiv. https://doi.org/10.1101/331496
Omachi K, Kamura M, Teramoto K, Kojima H, Yokota T, Kaseda S, Kuwazuru J, Fukuda R, Koyama K, Matsuyama S, Motomura K, Shuto T, Suico MA, Kai H (2018) A split-luciferase-based trimer formation assay as a high-throughput screening platform for therapeutics in alport syndrome. Cell Chem Biol 25(634–43):e4. https://doi.org/10.1016/j.chembiol.2018.02.003
Wong MY, Doan ND, DiChiara AS, Papa LJ 3rd, Cheah JH, Soule CK, Watson N, Hulleman JD, Shoulders MD (2018) A high-throughput assay for collagen secretion suggests an unanticipated role for Hsp90 in collagen production. Biochemistry 57:2814–2827. https://doi.org/10.1021/acs.biochem.8b00378
Bonfanti L, Mironov AA Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A (1998) Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell 95:993–1003. https://doi.org/10.1016/s0092-8674(00)81723-7
Mironov AA, Mironov AA Jr, Beznoussenko GV, Trucco A, Lupetti P, Smith JD, Geerts WJ, Koster AJ, Burger KN, Martone ME, Deerinck TJ, Ellisman MH, Luini A (2003) ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev Cell 5:583–594. https://doi.org/10.1016/s1534-5807(03)00294-6
Weigel AV, Chang CL, Shtengel G, Xu CS, Hoffman DP, Freeman M, Iyer N, Aaron J, Khuon S, Bogovic J, Qiu W, Hess HF, Lippincott-Schwartz J (2021) ER-to-Golgi protein delivery through an interwoven, tubular network extending from ER. Cell 184(2412–29):e16. https://doi.org/10.1016/j.cell.2021.03.035
Zhang Y, Stefanovic B (2016) LARP6 meets collagen mRNA: specific regulation of type I collagen expression. Int J Mol Sci 17:419. https://doi.org/10.3390/ijms17030419
Chessler SD, Byers PH (1993) BiP binds type I procollagen pro alpha chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J Biol Chem 268:18226–18233. https://doi.org/10.1016/S0021-9258(17)46834-7
Scheiber AL, Guess AJ, Kaito T, Abzug JM, Enomoto-Iwamoto M, Leikin S, Iwamoto M, Otsuru S (2019) Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem Biophys Res Commun 509:235–240. https://doi.org/10.1016/j.bbrc.2018.12.111
Besio R, Iula G, Garibaldi N, Cipolla L, Sabbioneda S, Biggiogera M, Marini JC, Rossi A, Forlino A (2018) 4-PBA ameliorates cellular homeostasis in fibroblasts from osteogenesis imperfecta patients by enhancing autophagy and stimulating protein secretion. Biochim Biophys Acta Mol Basis Dis 1864:1642–1652. https://doi.org/10.1016/j.bbadis.2018.02.002
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. https://doi.org/10.1038/nprot.2013.143
Zhang JP, Li XL, Li GH, Chen W, Arakaki C, Botimer GD et al (2017) Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol 18:35. https://doi.org/10.1186/s13059-017-1164-8
Makareeva E, Cabral WA, Marini JC, Leikin S (2006) Molecular mechanism of alpha 1(I)-osteogenesis imperfecta/ehlers-danlos syndrome: unfolding of an N-anchor domain at the N-terminal end of the type I collagen triple helix. J Biol Chem 281:6463–6470. https://doi.org/10.1074/jbc.M511830200
Makareeva E, Sun G, Mirigian LS, Mertz EL, Vera JC, Espinoza NA, Yang K, Chen D, Klein TE, Byers PH, Leikin S (2018) Substitutions for arginine at position 780 in triple helical domain of the alpha1(I) chain alter folding of the type I procollagen molecule and cause osteogenesis imperfecta. PLoS One 13:e0200264. https://doi.org/10.1371/journal.pone.0200264
Leikina E, Mertts MV, Kuznetsova N, Leikin S (2002) Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci USA 99:1314–1318. https://doi.org/10.1073/pnas.032307099
Mirigian LS, Makareeva E, Leikin S (2014) Pulse-chase analysis of procollagen biosynthesis by azidohomoalanine labeling. Connect Tissue Res 55:403–410. https://doi.org/10.3109/03008207.2014.959120
Miura K (2020) Bleach correction ImageJ plugin for compensating the photobleaching of time-lapse sequences. F100Research. https://doi.org/10.12688/f1000research.27171.1
Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, Leikin S (2010) Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res 70:4366–4374. https://doi.org/10.1158/0008-5472.CAN-09-4057
Miles CA, Sims TJ, Camacho NP, Bailey AJ (2002) The role of the α2 chain in the stabilization of the collagen type I heterotrimer: a study of the type I homotrimer in oim mouse tissues. J Mol Biol 321:797–805. https://doi.org/10.1016/S0022-2836(02)00703-9
Kuznetsova NV, McBride DJ, Leikin S (2003) Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. J Mol Biol 331:191–200. https://doi.org/10.1016/s0022-2836(03)00715-0
Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW (1997) Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys J 73:2782–2790. https://doi.org/10.1016/S0006-3495(97)78307-3
Sengupta P, Satpute-Krishnan P, Seo AY, Burnette DT, Patterson GH, Lippincott-Schwartz J (2015) ER trapping reveals Golgi enzymes continually revisit the ER through a recycling pathway that controls Golgi organization. Proc Natl Acad Sci USA 112:E6752–E6761. https://doi.org/10.1073/pnas.1520957112
Acknowledgements
This work was funded by the Intramural Research Program of NICHD, NIH. The authors thank Juan Bonifacino (NICHD) and Jennifer Lippincott-Schwartz (Howard Hughes Medical Institute, Janelia Research Campus) for access to super-resolution microscopes in their laboratories. They also thank Vincent Schram for assistance in imaging at NICHD Microscopy and Imaging Core.
Funding
This work was funded by the Intramural Research Program of NICHD, NIH.
Author information
Authors and Affiliations
Contributions
LG, SO, EM, and SL contributed to Study design; LG, SO, and EM performed experiments; LG, SO, EM, and SL performed data analysis; LG, SO, EM, and SL were involved in writing and revising the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 1431 KB)
Supplementary file3 (MP4 8983 KB)
Supplementary file4 (MP4 7795 KB)
Supplementary file1 (MP4 17346 KB)
Rights and permissions
About this article
Cite this article
Gorrell, L., Omari, S., Makareeva, E. et al. Noncanonical ER–Golgi trafficking and autophagy of endogenous procollagen in osteoblasts. Cell. Mol. Life Sci. 78, 8283–8300 (2021). https://doi.org/10.1007/s00018-021-04017-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-04017-z