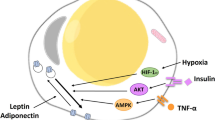
Abstract
The aim of the present study was to determine the role of Akt isoforms in insulin signaling and resistance in neuronal cells. By silencing Akt isoforms individually and in pairs, in Neuro-2a and HT22 cells we observed that, in insulin-sensitive condition, Akt isoforms differentially reduced activation of AS160 and glucose uptake with Akt2 playing the major role. Under insulin-resistant condition, phosphorylation of all isoforms and glucose uptake were severely affected. Over-expression of individual isoforms in insulin-sensitive and resistant cells differentially reversed AS160 phosphorylation with concomitant reversal in glucose uptake indicating a compensatory role of Akt isoforms in controlling neuronal insulin signaling. Post-insulin stimulation Akt2 translocated to the membrane the most followed by Akt3 and Akt1, decreasing glucose uptake in the similar order in insulin-sensitive cells. None of the Akt isoforms translocated in insulin-resistant cells or high-fat-diet mediated diabetic mice brain cells. Based on our data, insulin-dependent differential translocation of Akt isoforms to the plasma membrane turns out to be the key factor in determining Akt isoform specificity. Thus, isoforms play parallel with predominant role by Akt2, and compensatory yet novel role by Akt1 and Akt3 to regulate neuronal insulin signaling, glucose uptake, and insulin-resistance.













Similar content being viewed by others
Data availability
All the data related to this manuscript are available with the first author and corresponding author.
Code availability
Not applicable.
References
Gonzalez E, McGraw TE (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8:2502–2508
Hay N (2011) Akt isoforms and glucose homeostasis—the leptin connection. Trends Endocrinol Metab 22:66–73
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169:381–405
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14:1483–1496
Gupta A, Bisht B, Dey CS (2012) Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim Biophys Acta 1822:1030–1037
Gupta A, Dey CS (2012) PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell 23:3882–3898
de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2:1101–1113
Gupta A, Bisht B, Dey CS (2011) Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology 60:910–920
Humbert S, Bryson EA, Cordelières FP, Connors NC, Datta SR, Finkbeiner S et al (2002) The IGF-1/Akt pathway is neuroprotective in huntington’s disease and involves huntingtin phosphorylation by Akt. Dev Cell 2:831–837
Chen H-K, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH et al (2003) Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell 113:457–468
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004) Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36:131–137
Furlong RM, Lindsay A, Anderson KE, Hawkins PT, Sullivan AM, O’Neill C (2019) The Parkinson’s disease gene PINK1 activates Akt via PINK1 kinase-dependent regulation of the phospholipid PI(3,4,5)P3. J Cell Sci 132:jcs233221
Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW (2007) Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol 21:215–228
Kajno E, McGraw TE, Gonzalez E (2015) Development of a new model system to dissect isoform specific Akt signalling in adipocytes. Biochem J 468:425–434
Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S et al (2012) Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 18:388–395
Tschopp O, Yang Z-Z, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T et al (2005) Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954
Chen WS, Xu P-Z, Gottlob K, Chen M-L, Sokol K, Shiyanova T et al (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev 15:2203–2208
Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ (2001) Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276:38349–38352
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB et al (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728–1731
Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL et al (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112:197–208
Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS et al (2005) Role for Akt3/protein kinase bγ in attainment of normal brain size. Mol Cell Biol 25:1869–1878
Lee RS, House CM, Cristiano BE, Hannan RD, Pearson RB, Hannan KM (2011) Relative expression levels rather than specific activity plays the major role in determining in vivo AKT isoform substrate specificity. Enzyme Res 2011:720985
Gonzalez E, McGraw TE (2009) Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling PNAS. Nat Acad Sci 106:7004–7009
Ding J, Du K (2009) ClipR-59 interacts with Akt and regulates Akt cellular compartmentalization. Mol Cell Biol 29:1459–1471
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P et al (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551
Sharma M, Dey CS (2021) AKT ISOFORMS-AS160-GLUT4: the defining axis of insulin resistance. Rev Endocr Metab Disord. https://doi.org/10.1007/s11154-021-09652-2 (Epub ahead of print. PMID: 33928491)
Bae SS, Cho H, Mu J, Birnbaum MJ (2003) Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278:49530–49536
Dummler B, Tschopp O, Hynx D, Yang Z-Z, Dirnhofer S, Hemmings BA (2006) Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26:8042–8051
George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC et al (2004) A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304:1325–1328
Noda S, Kishi K, Yuasa T, Hayashi H, Ohnishi T, Miyata I et al (2000) Overexpression of wild-type Akt1 promoted insulin-stimulated p70S6 kinase (p70S6K) activity and affected GSK3 beta regulation, but did not promote insulin-stimulated GLUT4 translocation or glucose transport in L6 myotubes. J Med Invest 47:47–55
Chen WS, Peng X-D, Wang Y, Xu P-Z, Chen M-L, Luo Y et al (2009) Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol 29:3151–3162
Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP (2003) Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100:7569–7574
Hajduch E, Alessi DR, Hemmings BA, Hundal HS (1998) Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47:1006–1013
Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA (2017) AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife. https://doi.org/10.7554/eLife.30640
Gabbouj S, Natunen T, Koivisto H, Jokivarsi K, Takalo M, Marttinen M et al (2019) Intranasal insulin activates Akt2 signaling pathway in the hippocampus of wild-type but not in APP/PS1 Alzheimer model mice. Neurobiol Aging 75:98–108
Diez H, Garrido JJ, Wandosell F (2012) Specific Roles of Akt iso Forms in Apoptosis and Axon Growth Regulation in Neurons. PLoS ONE 7:e32715
Schrötter S, Leondaritis G, Eickholt BJ (2016) Capillary isoelectric focusing of Akt isoforms identifies highly dynamic phosphorylation in neuronal cells and brain tissue. J Biol Chem 291:10239–10251
Arora A, Dey CS (2014) SIRT2 negatively regulates insulin resistance in C2C12 skeletal muscle cells. Biochim Biophys Acta 1842:1372–1378
Varshney P, Dey CS (2016) P21-activated kinase 2 (PAK2) regulates glucose uptake and insulin sensitivity in neuronal cells. Mol Cell Endocrinol 429:50–61
Liu J, Li L, Suo WZ (2009) HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci 84:267–271
Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS et al (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278:14599–14602
Ng Y, Ramm G, Burchfield JG, Coster ACF, Stöckli J, James DE (2010) Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J Biol Chem 285:2245–2257
Zheng X, Cartee GD (2016) Insulin-induced effects on the subcellular localization of AKT1, AKT2 and AS160 in rat skeletal muscle. Sci Rep 6:39230
Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V et al (2005) Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 280:37803–37813
Stöckli J, Davey JR, Hohnen-Behrens C, Xu A, James DE, Ramm G (2008) Regulation of glucose transporter 4 translocation by the Rab guanosine triphosphatase-activating protein AS160/TBC1D4: role of phosphorylation and membrane association. Mol Endocrinol 22:2703–2715
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5:214
Sirover MA (2012) Subcellular dynamics of multifunctional protein regulation: mechanisms of GAPDH intracellular translocation. J Cell Biochem 113:2193–2200
Arora A, Dey CS (2016) SIRT2 regulates insulin sensitivity in insulin resistant neuronal cells. Biochem Biophys Res Commun 474:747–752
Mishra D, Dey CS (2019) Protein kinase C attenuates insulin signalling cascade in insulin-sensitive and insulin-resistant neuro-2a cells. J Mol Neurosci 69:470–477
Varshney P, Dey CS (2017) Resveratrol regulates neuronal glucose uptake and insulin sensitivity via P21-activated kinase 2 (PAK2). Biochem Biophys Res Commun 485:372–378
Cozzone D, Fröjdö S, Disse E, Debard C, Laville M, Pirola L et al (2008) Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 51:512–521
Brozinick JT, Roberts BR, Dohm GL (2003) Defective signaling through Akt-2 and -3 But Not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes Am Diabetes Assoc 52:935–941
Wang L, Cheng S, Yin Z, Xu C, Lu S, Hou J et al (2015) Conditional inactivation of Akt three isoforms causes tau hyperphosphorylation in the brain. Mol Neurodegener 10:33
Tan S-X, Ng Y, Burchfield JG, Ramm G, Lambright DG, Stöckli J et al (2012) The Rab GTPase-activating protein TBC1D4/AS160 contains an atypical phosphotyrosine-binding domain that interacts with plasma membrane phospholipids to facilitate GLUT4 trafficking in adipocytes. Mol Cell Biol 32:4946–4959
Ramm G, Larance M, Guilhaus M, James DE (2006) A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281:29174–29180
Bruss MD, Arias EB, Lienhard GE, Cartee GD (2005) Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54:41–50
Kane S, Sano H, Liu SCH, Asara JM, Lane WS, Garner CC et al (2002) A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277:22115–22118
Zeigerer A, McBrayer MK, McGraw TE (2004) Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell 15:4406–4415
Brewer PD, Romenskaia I, Kanow MA, Mastick CC (2011) Loss of AS160 Akt substrate causes Glut4 protein to accumulate in compartments that are primed for fusion in basal adipocytes. J Biol Chem 286:26287–26297
Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE et al (2005) Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2:263–272
Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE et al (2006) Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55:2067–2076
Lansey MN, Walker NN, Hargett SR, Stevens JR, Keller SR (2012) Deletion of Rab GAP AS160 modifies glucose uptake and GLUT4 translocation in primary skeletal muscles and adipocytes and impairs glucose homeostasis. Am J Physiol Endocrinol Metab 303:E1273–E1286
Gosmanov AR, Umpierrez GE, Karabell AH, Cuervo R, Thomason DB (2004) Impaired expression and insulin-stimulated phosphorylation of Akt-2 in muscle of obese patients with atypical diabetes. Am J Physiol Endocrinol Metab 287:E8-15
Kim YB, Peroni OD, Franke TF, Kahn BB (2000) Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats. Diabetes 49:847–856
Heide LPVD, Hoekman MF, Biessels GJ, Gispen WH (2003) Insulin inhibits extracellular regulated kinase 1/2 phosphorylation in a phosphatidylinositol 3-kinase (PI3) kinase-dependent manner in Neuro2a cells. J Neurochem 86:86–91
Bisht B, Dey CS (2008) Focal Adhesion Kinase contributes to insulin-induced actin reorganization into a mesh harboring Glucose transporter-4 in insulin resistant skeletal muscle cells. BMC Cell Biol 9:48
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Goel HL, Dey CS (2002) Insulin stimulates spreading of skeletal muscle cells involving the activation of focal adhesion kinase, phosphatidylinositol 3-kinase and extracellular signal regulated kinases. J Cell Physiol 193:187–198
Silva G, Ferraresi C, de Almeida RT, Motta ML, Paixão T, Ottone VO et al (2020) Insulin resistance is improved in high-fat fed mice by photobiomodulation therapy at 630 nm. J Biophotonics 13:e201960140
Mujawdiya PK, Sharma P, Sharad S, Kapur S (2020) Reversal of increase in intestinal permeability by mangifera indica seed kernel extract in high-fat diet-induced obese mice. Pharmaceuticals (Basel). https://doi.org/10.3390/ph13080190
Acknowledgements
We are grateful to Indian Institute of Technology-Delhi for their support. We would like to thank Dr. Prosenjit Mondal, Indian Institute of Technology—Mandi, Himachal Pradesh, India, for helping us in providing with normal diet and high-fat-fed diet mice whole brain samples. We would like to thank Dr. Nishi Raj, Jamia Hamdard-Institute of Molecular Medicine (JHIMM), New Delhi, India for providing pCMV6 Empty vector backbone. We would like to thank Dr. Chinmoy Mukhopadhyay, Jawaharalal Nehru University, New Delhi, for providing us with Caveolin-1 antibody. We would like to thank Dr. Pallavi Varshney for her assistance in training some methodologies. We are grateful to Advanced Technology Platform Centre, Regional Centre for Biotechnology, Faridabad, Haryana, India, for Confocal Microscopy.
Funding
MS is a recipient of Senior Research Fellowship (09/086 (1256)/2016-EMR-1) from Council of Scientific and Industrial Research (CSIR), Government of India. CSD is supported by a grant from Department of Biotechnology, Government of India, New Delhi, India (PR32795/MED/122/229/2019).
Author information
Authors and Affiliations
Contributions
MS performed all the experiments, analyzed the data and wrote the original drafts, approved the final version of the manuscript. CSD conceived the idea, provided resources, acquired the funding, written, reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Mice whole brain tissues were kind gift from Dr. Prosenjit Mondal, Indian Institute of Technology—Mandi, HP, India. All experiments were performed following the guidelines prescribed by CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) with the approval of the Internal Animal Ethics Committee, Visva-Bharati (IAEC/VB/2017/01). The legal requirements/guidelines in the country for the care and use of animals have been followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, M., Dey, C.S. Role of Akt isoforms in neuronal insulin signaling and resistance. Cell. Mol. Life Sci. 78, 7873–7898 (2021). https://doi.org/10.1007/s00018-021-03993-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03993-6