Abstract
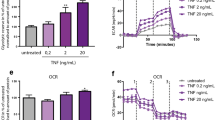
Recent studies have shown the significance of metabolic reprogramming in immune and stromal cell function. Yet, the metabolic reconfiguration of RA macrophages (MΦs) is incompletely understood during active disease and in crosstalk with other cell types in experimental arthritis. This study elucidates a distinct regulation of glycolysis and oxidative phosphorylation in RA MΦs compared to fibroblast (FLS), although PPP (Pentose Phosphate pathway) is similarly reconfigured in both cell types. 2-DG treatment showed a more robust impact on impairing the RA M1 MΦ-mediated inflammatory phenotype than IACS-010759 (IACS, complexli), by reversing ERK, AKT and STAT1 signaling, IRF8/3 transcription and CCL2 or CCL5 secretion. This broader inhibitory effect of 2-DG therapy on RA M1 MΦs was linked to dysregulation of glycolysis (GLUT1, PFKFB3, LDHA, lactate) and oxidative PPP (NADP conversion to NADPH), while both compounds were ineffective on oxidative phosphorylation. Distinctly, in RA FLS, 2-DG and IACS therapies constrained LPS/IFNγ-induced AKT and JNK signaling, IRF5/7 and fibrokine expression. Disruption of RA FLS metabolic rewiring by 2-DG or IACS therapy was accompanied by a reduction of glycolysis (HIF1α, PFKFB3) and suppression of citrate or succinate buildup. We found that 2-DG therapy mitigated CIA pathology by intercepting joint F480+iNOS+MΦ, Vimentin+ fibroblast and CD3+T cell trafficking along with downregulation of IRFs and glycolytic intermediates. Surprisingly, IACS treatment was inconsequential on CIA swelling, cell infiltration, M1 and Th1/Th17 cytokines (IFN-γ/IL-17) and joint glycolytic mediators. Collectively, our results indicate that blockade of glycolysis is more effective than inhibition of complex 1 in CIA, in part due to its effectiveness on the MΦ inflammatory phenotype.








Similar content being viewed by others
References
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389(10086):2328–2337
Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S (2017) TLRs, future potential therapeutic targets for RA. Autoimmun Rev 16(2):103–113
Kelly B, O’Neill LA (2015) Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25(7):771–784
O’Neill LA (2015) A broken krebs cycle in macrophages. Immunity 42(3):393–394
Biniecka M, Canavan M, McGarry T, Gao W, McCormick J, Cregan S, Gallagher L, Smith T, Phelan JJ, Ryan J, O’Sullivan J, Ng CT, Veale DJ, Fearon U (2016) Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis 75(12):2192–2200
Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, Boss GR, Tiziani S, Murphy AN, Guma M (2016) Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol 68(7):1614–1626
Fearon U, Hanlon MM, Wade SM, Fletcher JM (2019) Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin Exp Immunol 197(2):170–180
de Oliveira PG, Farinon M, Sanchez-Lopez E, Miyamoto S, Guma M (2019) Fibroblast-like synoviocytes glucose metabolism as a therapeutic target in rheumatoid arthritis. Front Immunol 10:1743
Wen Z, Jin K, Shen Y, Yang Z, Li Y, Wu B, Tian L, Shoor S, Roche NE, Goronzy JJ, Weyand CM (2019) N-myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat Immunol 20(3):313–325
Shen Y, Wen Z, Li Y, Matteson EL, Hong J, Goronzy JJ, Weyand CM (2017) Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat Immunol 18(9):1025–1034
Weyand CM, Goronzy JJ (2021) The immunology of rheumatoid arthritis. Nat Immunol 22:10–18
Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Goodman SM, Tabechian D, Hughes LB, Salomon-Escoto K, Watts GFM, Jonsson AH, Rangel-Moreno J, Meednu N, Rozo C, Apruzzese W, Eisenhaure TM, Lieb DJ, Boyle DL, Mandelin AM 2nd, Accelerating Medicines Partnership Rheumatoid A, Systemic Lupus Erythematosus C, Boyce BF, DiCarlo E, Gravallese EM, Gregersen PK, Moreland L, Firestein GS, Hacohen N, Nusbaum C, Lederer JA, Perlman H, Pitzalis C, Filer A, Holers VM, Bykerk VP, Donlin LT, Anolik JH, Brenner MB, Raychaudhuri S (2019) Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol 20(7):928–942
McGarry T, Orr C, Wade S, Biniecka M, Wade S, Gallagher L, Low C, Veale DJ, Fearon U (2018) JAK/STAT blockade alters synovial bioenergetics, mitochondrial function, and proinflammatory mediators in rheumatoid arthritis. Arthritis Rheumatol 70(12):1959–1970
Shervington L, Darekar A, Shaikh M, Mathews R, Shervington A (2018) Identifying reliable diagnostic/predictive biomarkers for rheumatoid arthritis. Biomark Insights 13:1177271918801005
Zou Y, Zeng S, Huang M, Qiu Q, Xiao Y, Shi M, Zhan Z, Liang L, Yang X, Xu H (2017) Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br J Pharmacol 174(9):893–908
Bustamante MF, Oliveira PG, Garcia-Carbonell R, Croft AP, Smith JM, Serrano RL, Sanchez-Lopez E, Liu X, Kisseleva T, Hay N, Buckley CD, Firestein GS, Murphy AN, Miyamoto S, Guma M (2018) Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis 77(11):1636–1643
Veras FP, Peres RS, Saraiva AL, Pinto LG, Louzada-Junior P, Cunha TM, Paschoal JA, Cunha FQ, Alves-Filho JC (2015) Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci Rep 5:15171
Guma M, Wang Y, Viollet B, Liu-Bryan R (2015) AMPK Activation by A-769662 Controls IL-6 expression in inflammatory arthritis. PLoS ONE 10(10):e0140452
Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, McAfoos T, Morlacchi P, Ackroyd J, Agip AA, Al-Atrash G, Asara J, Bardenhagen J, Carrillo CC, Carroll C, Chang E, Ciurea S, Cross JB, Czako B, Deem A, Daver N, de Groot JF, Dong JW, Feng N, Gao G, Gay J, Do MG, Greer J, Giuliani V, Han J, Han L, Henry VK, Hirst J, Huang S, Jiang Y, Kang Z, Khor T, Konoplev S, Lin YH, Liu G, Lodi A, Lofton T, Ma H, Mahendra M, Matre P, Mullinax R, Peoples M, Petrocchi A, Rodriguez-Canale J, Serreli R, Shi T, Smith M, Tabe Y, Theroff J, Tiziani S, Xu Q, Zhang Q, Muller F, DePinho RA, Toniatti C, Draetta GF, Heffernan TP, Konopleva M, Jones P, Di Francesco ME, Marszalek JR (2018) An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med 24(7):1036–1046
Zhang L, Yao Y, Zhang S, Liu Y, Guo H, Ahmed M, Bell T, Zhang H, Han G, Lorence E, Badillo M, Zhou S, Sun Y, Di Francesco ME, Feng N, Haun R, Lan R, Mackintosh SG, Mao X, Song X, Zhang J, Pham LV, Lorenzi PL, Marszalek J, Heffernan T, Draetta G, Jones P, Futreal A, Nomie K, Wang L, Wang M (2019) Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med 11(491):eaau1167
Tsuji A, Akao T, Masuya T, Murai M, Miyoshi H (2020) IACS-010759, a potent inhibitor of glycolysis-deficient hypoxic tumor cells, inhibits mitochondrial respiratory complex I through a unique mechanism. J Biol Chem 295(21):7481–7491
Souto-Carneiro MM, Klika KD, Abreu MT, Meyer AP, Saffrich R, Sandhoff R, Jennemann R, Kraus FV, Tykocinski L, Eckstein V, Carvalho L, Kriegsmann M, Giese T, Lorenz HM, Carvalho RA (2020) Effect of increased lactate dehydrogenase A activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+ T cells in rheumatoid arthritis. Arthritis Rheumatol 72(12):2050–2064
Ahn JK, Kim J, Cheong YE, Kim KH, Cha HS (2020) Variation in the synovial fluid metabolome according to disease activity of rheumatoid arthritis. Clin Exp Rheumatol 38(3):500–507
Van Linthoudt D, Salani I, Zender R, Locatelli P, Ott H, Schumacher HR Jr (1996) Citrate in synovial fluid and its relation to inflammation and crystal presence. J Rheumatol 23(3):502–505
Buldak L, Machnik G, Buldak RJ, Labuzek K, Boldys A, Okopien B (2016) Exenatide and metformin express their anti-inflammatory effects on human monocytes/macrophages by the attenuation of MAPKs and NFkappaB signaling. Naunyn Schmiedebergs Arch Pharmacol 389(10):1103–1115
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271(51):32529–32537
Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A (2001) Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 276(12):9519–9525
van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J 412(3):477–484
Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C (2012) Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA 109(24):9517–9522
Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG (2014) mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345(6204):1250684
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3(3):177–185
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DGW, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O’Neill LAJ (2015) Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab 21(2):347
Tan Z, Xie N, Banerjee S, Cui H, Fu M, Thannickal VJ, Liu G (2015) The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem 290(1):46–55
Chen CL, Cheng MH, Kuo CF, Cheng YL, Li MH, Chang CP, Wu JJ, Anderson R, Wang S, Tsai PJ, Liu CC, Lin YS (2018) Dextromethorphan attenuates NADPH oxidase-regulated glycogen synthase kinase 3beta and NF-kappaB activation and reduces nitric oxide production in group A streptococcal infection. Antimicrob Agents Chemother 62(6):e02045–e2117
West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472(7344):476–480
Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42(3):419–430
Meiser J, Kramer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, Skupin A, Hiller K (2016) Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem 291(8):3932–3946
Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA 110(19):7820–7825
Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, Metallo CM (2016) Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem 291(27):14274–14284
Bartok B, Boyle DL, Liu Y, Ren P, Ball ST, Bugbee WD, Rommel C, Firestein GS (2012) PI3 kinase delta is a key regulator of synoviocyte function in rheumatoid arthritis. Am J Pathol 180(5):1906–1916
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39(2):171–183
Bradley K, Scatizzi JC, Fiore S, Shamiyeh E, Koch AE, Firestein GS, Gorges LL, Kuntsman K, Pope RM, Moore TL, Han J, Perlman H (2004) Retinoblastoma suppression of matrix metalloproteinase 1, but not interleukin-6, through a p38-dependent pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 50(1):78–87
Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS (1998) NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA 95(23):13859–13864
Weyand CM, Goronzy JJ (2017) Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol 13(5):291–301
Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM (2013) Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med 210(10):2119–2134
Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y, Huang L, Chen Z, Zheng J, Yang C (2019) Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res 11(4):2393–2402
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186(6):3299–3303
Abboud G, Choi SC, Kanda N, Zeumer-Spataro L, Roopenian DC, Morel L (2018) Inhibition of glycolysis reduces disease severity in an autoimmune model of rheumatoid arthritis. Front Immunol 9:1973
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TAJ, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Koch AE, Polverini PJ, Leibovich SJ (1986) Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum 29(4):471–479
Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM 2nd, Shahrara S (2011) Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum 63(4):914–922
Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM 2nd, Shahrara S (2011) Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 63(10):2884–2893
Palasiewicz K, Umar S, Romay B, Zomorrodi RK, Shahrara S (2021) Tofacitinib therapy intercepts macrophage metabolic reprogramming instigated by SARS-CoV-2 Spike protein. Eur J Immunol 51:2330–2340
Van Raemdonck K, Umar S, Palasiewicz K, Volin MV, Elshabrawy HA, Romay B, Tetali C, Ahmed A, Amin MA, Zomorrodi RK, Sweiss N, Shahrara S (2021) IL-34 reprograms glycolytic and osteoclastic RA macrophages via Syndecan-1 and M-CSFR. Arthritis Rheumatol. https://doi.org/10.1002/art.41792
Pickens SR, Chamberlain ND, Volin MV, Mandelin AM 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S (2011) Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum 63(8):2289–2298
Chen Z, Kim SJ, Chamberlain ND, Pickens SR, Volin MV, Volkov S, Arami S, Christman JW, Prabhakar BS, Swedler W, Mehta A, Sweiss N, Shahrara S (2013) The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis. J Immunol 190(10):5256–5266
Kim SJ, Chen Z, Essani AB, Elshabrawy HA, Volin MV, Fantuzzi G, McInnes IB, Baker JF, Finn P, Kondos G, Volkov S, Swedler W, Arami S, Sweiss N, Shahrara S (2017) Differential impact of obesity on the pathogenesis of RA or preclinical models is contingent on the disease status. Ann Rheum Dis 76(4):731–739
Umar S, Palasiewicz K, Van Raemdonck K, Volin MV, Romay B, Amin MA, Zomorrodi RK, Arami S, Gonzalez M, Rao V, Zanotti B, Fox DA, Sweiss N, Shahrara S (2021) IRAK4 inhibition: a promising strategy for treating RA joint inflammation and bone erosion. Cell Mol Immunol 18:2199–2210
Kim SJ, Chen Z, Chamberlain ND, Volin MV, Swedler W, Volkov S, Sweiss N, Shahrara S (2013) Angiogenesis in rheumatoid arthritis is fostered directly by toll-like receptor 5 ligation and indirectly through interleukin-17 induction. Arthritis Rheum 65(8):2024–2036
Kim SJ, Chen Z, Chamberlain ND, Essani AB, Volin MV, Amin MA, Volkov S, Gravallese EM, Arami S, Swedler W, Lane NE, Mehta A, Sweiss N, Shahrara S (2014) Ligation of TLR5 promotes myeloid cell infiltration and differentiation into mature osteoclasts in rheumatoid arthritis and experimental arthritis. J Immunol 193(8):3902–3913
Funding
This work was supported in part by awards from the Department of Veteran’s Affairs MERIT Award BX002286, the National Institutes of Health NIH AI147697, the National Psoriasis Foundation (NPF), Pfizer Investigator-Initiated Research (IIR) Program and Chicago Biomedical Consortium (CBC) Accelerator Award.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. SS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design. SU, SS. Acquisition of data. SU, KP, MVV, BR, RR, CT, RZ, SS. Analysis and interpretation of data. SU, KP, MVV, BR, RR, CT, MG, CA, RZ, LO, SS. Providing crucial reagents. SA, NS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no commercial or financial conflict of interest exists.
Ethics approval and consent to participate
All peripheral blood (PB) was collected in accordance with our protocol approved by the University of Illinois at Chicago Institutional Ethics Review Board. All the RA patients have consented to participate in this study and have provided written consent. Furthermore, all animal studies were approved by the University of Illinois at Chicago Animal Care and Use Committee following the legal requirements and guidelines of the state of Illinois in the USA and NIH.
Author’s consent to publication
All authors have been involved in writing the manuscript and consented to publication.
Availability of data and material
All findings are exhibited in the paper and the material and data are available for transparency.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2021_3978_MOESM1_ESM.tif
Supplementary file1 RA monocyte-differentiated MΦs were treated with PBS or LPS/IFNγ (100ng/ml each) for 24h before quantifying the F480+CD86+ cells or F480+CD206+ MΦs, (A1-A2, n=4). RA MΦs were pretreated with DMSO, 2-DG (5mM) or IACS (100nM) o/n. Thereafter cells were untreated (PBS) or stimulated with LPS/IFNγ (100ng/ml each) for 24h before quantifying pyruvate (Sigma MAK332; A3) via colorimetric assay (n=5) or for 6h before measuring HIF1α (A4) transcription by real-time RT-PCR, n=6. The data are shown as mean ± SEM, ** represents p<0.01 and **** denotes p<0.0001 (TIF 199 KB)
18_2021_3978_MOESM2_ESM.tif
Supplementary file2 Western blot density was quantified by Image J in Fig. 1A (B1) and Fig. 2M (B2) and was normalized to equal loading, n=3. The data are shown as mean ± SEM, * represents p<0.05 and ** denotes p<0.01 (TIF 135 KB)
18_2021_3978_MOESM3_ESM.tif
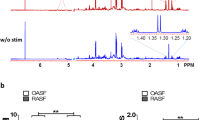
Supplementary file3 Western blot density was quantified by Image J in Fig. 3A (C1) and Fig. 4K (C2) and was normalized to equal loading, n=3. RA FLS were pretreated with DMSO, 2-DG (5mM) or IACS (100nM) o/n. Thereafter cells were untreated (PBS) or stimulated with LPS/IFNγ (100ng/ml each) for 24h before quantifying pyruvate (Sigma MAK332) via colorimetric assay (C3, n=5). To provide a rationale for signaling timepoint selection (30min), RA FLS were treated with LPS/IFNγ (100ng/ml each) for 0-60min and lysates were probed for pERK, pSTAT1and actin (C4). The data are shown as mean ± SEM, * represents p<0.05 and ** denotes p<0.01 (TIF 226 KB)
18_2021_3978_MOESM4_ESM.tif
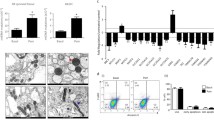
Supplementary file4 Images with higher magnification are provided for joint F480, iNOS and Arginase staining in CIA mice treated with control, 2-DG or IACS (D1). CIA ankles treated with control, 2-DG or IACS were immunostained with F480 red (1;100, Genetex), iNOS green (Santa Cruz, 1:200), Arginase 1 green (Santa Cruz, 1:200). Each tissue section was analyzed using Image J’s Color threshold function. This function was used to quantify the double-positive cells. 7-20 fields were quantified per tissue, depending upon tissue size and their integrated densities (product of area and mean gray value) and values were averaged (D2 and D3). The data are shown as mean ± SEM, * represents p<0.05 (TIF 2353 KB)
Rights and permissions
About this article
Cite this article
Umar, S., Palasiewicz, K., Volin, M.V. et al. Metabolic regulation of RA macrophages is distinct from RA fibroblasts and blockade of glycolysis alleviates inflammatory phenotype in both cell types. Cell. Mol. Life Sci. 78, 7693–7707 (2021). https://doi.org/10.1007/s00018-021-03978-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03978-5