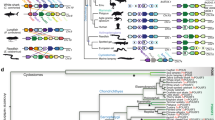
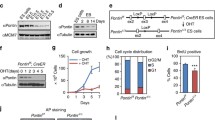
Abstract
Oct4, a class V POU-domain protein that is encoded by the Pou5f1 gene, is thought to be a key transcription factor in the early development of mammals. This transcription factor plays indispensable roles in pluripotent stem cells as well as in the acquisition of pluripotency during somatic cell reprogramming. Oct4 has also been shown to play a role as a pioneer transcription factor during zygotic genome activation (ZGA) from zebrafish to human. However, during the past decade, several studies have brought these conclusions into question. It was clearly shown that the first steps in mouse development are not affected by the loss of Oct4. Subsequently, the role of Oct4 as a genome activator was brought into doubt. It was also found that the reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) could proceed without Oct4. In this review, we summarize recent findings, reassess the role of Oct4 in reprogramming and ZGA, and point to structural features that may underlie this role. We speculate that pluripotent stem cells resemble neural stem cells more closely than previously thought. Oct4 orthologs within the POUV class hold key roles in genome activation during early development of species with late ZGA. However, in Placentalia, eutherian-specific proteins such as Dux overtake Oct4 in ZGA and endow them with the formation of an evolutionary new tissue—the placenta.




Similar content being viewed by others
Availability of data and material
Not applicable.
References
Schöler HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P (1989) A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J 8:2543–2550. https://doi.org/10.1002/j.1460-2075.1989.tb08392.x
Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM (1990) A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 345:686–692. https://doi.org/10.1038/345686a0
Herr W, Sturm RA, Clerc RG, Corcoran LM, Baltimore D, Sharp PA, Ingraham HA et al (1988) The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. https://doi.org/10.1101/gad.2.12a.1513
Botquin V, Hess H, Fuhrmann G, Anastassiadis C, Gross MK, Vriend G, Schöler HR (1998) New POU dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes Dev 12:2073–2090. https://doi.org/10.1101/gad.12.13.2073
Tomilin A, Remenyi A, Lins K, Bak H, Leidel S, Vriend G, Wilmanns M et al (2000) Synergism with the coactivator OBF-1 (OCA-B, BOB-1) is mediated by a specific POU dimer configuration. Cell. https://doi.org/10.1016/s0092-8674(00)00189-6
Remenyi A, Tomilin A, Pohl E, Lins K, Philippsen A, Reinbold R, Schöler HR et al (2001) Differential dimer activities of the transcription factor Oct-1 by DNA-induced interface swapping. Mol Cell 8:569–80. https://doi.org/10.1016/s1097-2765(01)00336-7
Remenyi A, Lins K, Nissen LJ, Reinbold R, Scholer HR, Wilmanns M (2003) Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev 17:2048–2059. https://doi.org/10.1101/gad.269303
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H et al (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391. https://doi.org/10.1016/s0092-8674(00)81769-9
Wu G, Han D, Gong Y, Sebastiano V, Gentile L, Singhal N, Adachi K et al (2013) Establishment of totipotency does not depend on Oct4A. Nat Cell Biol 15:1089–1097. https://doi.org/10.1038/ncb2816
Niwa H, Miyazaki J-I, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24:372–376. https://doi.org/10.1038/74199
Radzisheuskaya A, Chia Gle B, dos Santos RL, Theunissen TW, Castro LF, Nichols J, Silva JC (2013) A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat Cell Biol 15:579–590. https://doi.org/10.1038/ncb2742
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
An Z, Liu P, Zheng J, Si C, Li T, Chen Y, Ma T et al (2019) Sox2 and Klf4 as the functional core in pluripotency induction without exogenous Oct4. Cell Rep 29(1986–2000):e8. https://doi.org/10.1016/j.celrep.2019.10.026
Velychko S, Adachi K, Kim K-P, Hou Y, MacCarthy CM, Wu G, Schöler HR (2019) Excluding Oct4 from yamanaka cocktail unleashes the developmental potential of iPSCs. Cell Stem Cell. https://doi.org/10.1016/j.stem.2019.10.002
Michael AK, Grand RS, Isbel L, Cavadini S, Kozicka Z, Kempf G, Bunker RD et al (2020) Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Science. https://doi.org/10.1126/science.abb0074
Le Bin GC, Munoz-Descalzo S, Kurowski A, Leitch H, Lou X, Mansfield W, Etienne-Dumeau C et al (2014) Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development 141:1001–1010. https://doi.org/10.1242/dev.096875
Frum T, Halbisen MA, Wang C, Amiri H, Robson P, Ralston A (2013) Oct4 Cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev Cell 25:610–622. https://doi.org/10.1016/j.devcel.2013.05.004
Verrijzer CP, Van der Vliet PC (1993) POU domain transcription factors. Biochim Biophys Acta 1173:1–21. https://doi.org/10.1016/0167-4781(93)90237-8
Klemm JD, Rould MA, Aurora R, Herr W, Pabo CO (1994) Crystal structure of the Oct-1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Cell 77:21–32. https://doi.org/10.1016/0092-8674(94)90231-3
Pan G, Qin B, Liu N, Scholer HR, Pei D (2004) Identification of a nuclear localization signal in OCT4 and generation of a dominant negative mutant by its ablation. J Biol Chem 279:37013–37020. https://doi.org/10.1074/jbc.M405117200
Kong X, Liu J, Li L, Yue L, Zhang L, Jiang H, Xie X et al (2015) Functional interplay between the RK motif and linker segment dictates Oct4-DNA recognition. Nucleic Acids Res 43:4381–4392. https://doi.org/10.1093/nar/gkv323
Esch D, Vahokoski J, Groves MR, Pogenberg V, Cojocaru V, Vom Bruch H, Han D et al (2013) A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat Cell Biol 15:295–301. https://doi.org/10.1038/ncb2680
Roberts GA, Ozkan B, Gachulincova I, O’Dwyer MR, Hall-Ponsele E, Saxena M, Robinson PJ et al (2021) Dissecting OCT4 defines the role of nucleosome binding in pluripotency. Nat Cell Biol 23:834–845. https://doi.org/10.1038/s41556-021-00727-5
Jerabek S, Ng CKL, Wu G, Arauzo-Bravo MJ, Kim KP, Esch D, Malik V et al (2016) Changing POU dimerization preferences converts Oct6 into a pluripotency inducer. EMBO Rep 18:319–333. https://doi.org/10.15252/embr.201642958
Jacobson EM, Li P, Leon-del-Rio A, Rosenfeld MG, Aggarwal AK (1997) Structure of Pit-1 POU domain bound to DNA as a dimer: unexpected arrangement and flexibility. Genes Dev 11:198–212. https://doi.org/10.1101/gad.11.2.198
Malik V, Glaser LV, Zimmer D, Velychko S, Weng M, Holzner M, Arend M et al (2019) Pluripotency reprogramming by competent and incompetent POU factors uncovers temporal dependency for Oct4 and Sox2. Nat Commun 10:3477. https://doi.org/10.1038/s41467-019-11054-7
Tan DS, Chen Y, Gao Y, Bednarz A, Wei Y, Malik V, Ho DH et al (2021) Directed evolution of an enhanced POU reprogramming factor for cell fate engineering. Mol Biol Evol. https://doi.org/10.1093/molbev/msab075
Williams DC Jr, Cai M, Clore GM (2004) Molecular basis for synergistic transcriptional activation by Oct1 and Sox2 revealed from the solution structure of the 42-kDa Oct1.Sox2.Hoxb1-DNA ternary transcription factor complex. J Biol Chem 279:1449–1457. https://doi.org/10.1074/jbc.M309790200
Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F (2005) Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem 280:5307–5317. https://doi.org/10.1074/jbc.M410015200
Yuan H, Corbi N, Basilico C, Dailey L (1995) Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev 9:2635–2645. https://doi.org/10.1101/gad.9.21.2635
Nishimoto M, Fukushima A, Okuda A, Muramatsu M (1999) The gene for the embryonic stem cell coactivator UTF1 carries a regulatory element which selectively interacts with a complex composed of Oct-3/4 and Sox-2. Mol Cell Biol 19:5453–5465. https://doi.org/10.1128/MCB.19.8.5453
Tomioka M, Nishimoto M, Miyagi S, Katayanagi T, Fukui N, Niwa H, Muramatsu M et al (2002) Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res 30:3202–3213. https://doi.org/10.1093/nar/gkf435
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P (2005) Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem 280:24731–24737. https://doi.org/10.1074/jbc.M502573200
Tapia N, MacCarthy C, Esch D, Gabriele Marthaler A, Tiemann U, Arauzo-Bravo MJ, Jauch R et al (2015) Dissecting the role of distinct OCT4-SOX2 heterodimer configurations in pluripotency. Sci Rep 5:13533. https://doi.org/10.1038/srep13533
Velychko S, Kang K, Kim SM, Kwak TH, Kim KP, Park C, Hong K et al (2019) Fusion of reprogramming factors alters the trajectory of somatic lineage conversion. Cell Rep 27(30–39):e4. https://doi.org/10.1016/j.celrep.2019.03.023
Leichsenring M, Maes J, Mossner R, Driever W, Onichtchouk D (2013) Pou5f1 transcription factor controls zygotic gene activation in vertebrates. Science 341:1005–1009. https://doi.org/10.1126/science.1242527
Mistri TK, Devasia AG, Chu LT, Ng WP, Halbritter F, Colby D, Martynoga B et al (2015) Selective influence of Sox2 on POU transcription factor binding in embryonic and neural stem cells. EMBO Rep 16:1177–1191. https://doi.org/10.15252/embr.201540467
Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, Legant W et al (2014) Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156:1274–1285. https://doi.org/10.1016/j.cell.2014.01.062
Nishimoto M, Miyagi S, Yamagishi T, Sakaguchi T, Niwa H, Muramatsu M, Okuda A (2005) Oct-3/4 maintains the proliferative embryonic stem cell state via specific binding to a variant octamer sequence in the regulatory region of the UTF1 locus. Mol Cell Biol 25:5084–5094. https://doi.org/10.1128/MCB.25.12.5084-5094.2005
Pan X, Cang X, Dan S, Li J, Cheng J, Kang B, Duan X et al (2016) Site-specific disruption of the Oct4/Sox2 protein interaction reveals coordinated mesendodermal differentiation and the epithelial-mesenchymal transition. J Biol Chem 291:18353–18369. https://doi.org/10.1074/jbc.M116.745414
Aksoy I, Jauch R, Chen J, Dyla M, Divakar U, Bogu GK, Teo R et al (2013) Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J 32:938–953. https://doi.org/10.1038/emboj.2013.31
Jauch R, Aksoy I, Hutchins AP, Ng CK, Tian XF, Chen J, Palasingam P et al (2011) Conversion of Sox17 into a pluripotency reprogramming factor by reengineering its association with Oct4 on DNA. Stem Cells 29:940–951. https://doi.org/10.1002/stem.639
Merino F, Ng CKL, Veerapandian V, Scholer HR, Jauch R, Cojocaru V (2014) Structural basis for the SOX-dependent genomic redistribution of OCT4 in stem cell differentiation. Structure 22:1274–1286. https://doi.org/10.1016/j.str.2014.06.014
Niwa H, Sekita Y, Trend-Ayush E, Grützner F (2008) Platypus Pou5f1 reveals the first steps in the evolution of trophectoderm differentiation and pluripotency in mammals. Evol Dev 10:671–682. https://doi.org/10.1111/j.1525-142X.2008.00280.x
Lavial F, Acloque H, Bertocchini F, Macleod DJ, Boast S, Bachelard E, Montillet G et al (2007) The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 134:3549–3563. https://doi.org/10.1242/dev.006569
Morrison GM, Brickman JM (2006) Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development 133:2011–2022. https://doi.org/10.1242/dev.02362
Onichtchouk D, Geier F, Polok B, Messerschmidt DM, Mossner R, Wendik B, Song S et al (2010) Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol 6:354. https://doi.org/10.1038/msb.2010.9
Tapia N, Reinhardt P, Duemmler A, Wu G, Arauzo-Bravo MJ, Esch D, Greber B et al (2012) Reprogramming to pluripotency is an ancient trait of vertebrate Oct4 and Pou2 proteins. Nat Commun 3:1279. https://doi.org/10.1038/ncomms2229
Niwa H, Nakamura A, Urata M, Shirae-Kurabayashi M, Kuraku S, Russell S, Ohtsuka S (2016) The evolutionally-conserved function of group B1 Sox family members confers the unique role of Sox2 in mouse ES cells. BMC Evol Biol 16:173. https://doi.org/10.1186/s12862-016-0755-4
Gentsch GE, Spruce T, Owens NDL, Smith JC (2019) Maternal pluripotency factors initiate extensive chromatin remodelling to predefine first response to inductive signals. Nat Commun 10:4269. https://doi.org/10.1038/s41467-019-12263-w
Onichtchouk D, Driever W (2016) Zygotic genome activators, developmental timing, and pluripotency. Curr Top Dev Biol 116:273–297. https://doi.org/10.1016/bs.ctdb.2015.12.004
Okuda Y, Ogura E, Kondoh H, Kamachi Y (2010) B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet 6:e1000936. https://doi.org/10.1371/journal.pgen.1000936
Kobayashi K, Khan A, Ikeda M, Nakamoto A, Maekawa M, Yamasu K (2018) In vitro analysis of the transcriptional regulatory mechanism of zebrafish pou5f3. Exp Cell Res 364:28–41. https://doi.org/10.1016/j.yexcr.2018.01.023
Fernandez-Tresguerres B, Canon S, Rayon T, Pernaute B, Crespo M, Torroja C, Manzanares M (2010) Evolution of the mammalian embryonic pluripotency gene regulatory network. Proc Natl Acad Sci USA 107:19955–19960. https://doi.org/10.1073/pnas.1010708107
Han JY, Lee HG, Park YH, Hwang YS, Kim SK, Rengaraj D, Cho BW et al (2018) Acquisition of pluripotency in the chick embryo occurs during intrauterine embryonic development via a unique transcriptional network. J Anim Sci Biotechnol 9:31. https://doi.org/10.1186/s40104-018-0246-0
Brumbaugh J, Hou Z, Russell JD, Howden SE, Yu P, Ledvina AR, Coon JJ et al (2012) Phosphorylation regulates human OCT4. Proc Natl Acad Sci USA 109:7162–7168. https://doi.org/10.1073/pnas.1203874109
Saxe JP, Tomilin A, Scholer HR, Plath K, Huang J (2009) Post-translational regulation of Oct4 transcriptional activity. PLoS One 4:e4467. https://doi.org/10.1371/journal.pone.0004467
Abulaiti X, Zhang H, Wang A, Li N, Li Y, Wang C, Du X et al (2017) Phosphorylation of threonine(343) Is crucial for OCT4 interaction with SOX2 in the maintenance of mouse embryonic stem cell pluripotency. Stem Cell Rep 9:1630–1641. https://doi.org/10.1016/j.stemcr.2017.09.001
Bae KB, Yu DH, Lee KY, Yao K, Ryu J, Lim DY, Zykova TA et al (2017) Serine 347 phosphorylation by JNKs negatively regulates OCT4 protein stability in mouse embryonic stem cells. Stem Cell Rep 9:2050–2064. https://doi.org/10.1016/j.stemcr.2017.10.017
Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X, Zhou L et al (2012) Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell 48:627–640. https://doi.org/10.1016/j.molcel.2012.08.030
Spelat R, Ferro F, Curcio F (2012) Serine 111 phosphorylation regulates OCT4A protein subcellular distribution and degradation. J Biol Chem 287:38279–38288. https://doi.org/10.1074/jbc.M112.386755
Wei F, Scholer HR, Atchison ML (2007) Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. J Biol Chem 282:21551–21560. https://doi.org/10.1074/jbc.M611041200
Liao B, Jin Y (2010) Wwp2 mediates Oct4 ubiquitination and its own auto-ubiquitination in a dosage-dependent manner. Cell Res 20:332–344. https://doi.org/10.1038/cr.2009.136
Swaney DL, Wenger CD, Thomson JA, Coon JJ (2009) Human embryonic stem cell phosphoproteome revealed by electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci USA 106:995–1000. https://doi.org/10.1073/pnas.0811964106
Tolkunova E, Malashicheva A, Parfenov VN, Sustmann C, Grosschedl R, Tomilin A (2007) PIAS proteins as repressors of Oct4 function. J Mol Biol 374:1200–1212. https://doi.org/10.1016/j.jmb.2007.09.081
Jukam D, Shariati SAM, Skotheim JM (2017) Zygotic genome activation in vertebrates. Dev Cell 42:316–332. https://doi.org/10.1016/j.devcel.2017.07.026
Schulz KN, Harrison MM (2019) Mechanisms regulating zygotic genome activation. Nat Rev Genet 20:221–234. https://doi.org/10.1038/s41576-018-0087-x
Zaret KS, Carroll JS (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25:2227–2241. https://doi.org/10.1101/gad.176826.111
Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS (2015) Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161:555–568. https://doi.org/10.1016/j.cell.2015.03.017
Zhu F, Farnung L, Kaasinen E, Sahu B, Yin Y, Wei B, Dodonova SO et al (2018) The interaction landscape between transcription factors and the nucleosome. Nature 562:76–81. https://doi.org/10.1038/s41586-018-0549-5
Veil M, Yampolsky LY, Gruning B, Onichtchouk D (2019) Pou5f3, SoxB1, and Nanog remodel chromatin on high nucleosome affinity regions at zygotic genome activation. Genome Res 29:383–395. https://doi.org/10.1101/gr.240572.118
Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ (2013) Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 503:360–364. https://doi.org/10.1038/nature12632
Mirny LA (2010) Nucleosome-mediated cooperativity between transcription factors. Proc Natl Acad Sci USA 107:22534–22539. https://doi.org/10.1073/pnas.0913805107
Meers MP, Janssens DH, Henikoff S (2019) Pioneer factor-nucleosome binding events during differentiation are motif encoded. Mol Cell. https://doi.org/10.1016/j.molcel.2019.05.025
Chronis C, Fiziev P, Papp B, Butz S, Bonora G, Sabri S, Ernst J et al (2017) Cooperative binding of transcription factors orchestrates reprogramming. Cell. https://doi.org/10.1016/j.cell.2016.12.016
Liu G, Wang W, Hu S, Wang X, Zhang Y (2018) Inherited DNA methylation primes the establishment of accessible chromatin during genome activation. Genome Res 28:998–1007. https://doi.org/10.1101/gr.228833.117
King HW, Klose RJ (2017) The pioneer factor OCT4 requires the chromatin remodeller BRG1 to support gene regulatory element function in mouse embryonic stem cells. Elife. https://doi.org/10.7554/eLife.22631
Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E et al (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133:1106–1117. https://doi.org/10.1016/j.cell.2008.04.043
Stadhouders R, Vidal E, Serra F, Di Stefano B, Le Dily F, Quilez J, Gomez A et al (2018) Transcription factors orchestrate dynamic interplay between genome topology and gene regulation during cell reprogramming. Nat Genet 50:238–249. https://doi.org/10.1038/s41588-017-0030-7
Di Stefano B, Sardina JL, van Oevelen C, Collombet S, Kallin EM, Vicent GP, Lu J et al (2014) C/EBPalpha poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature 506:235–239. https://doi.org/10.1038/nature12885
Palfy M, Schulze G, Valen E, Vastenhouw NL (2020) Chromatin accessibility established by Pou5f3, Sox19b and Nanog primes genes for activity during zebrafish genome activation. PLoS Genet 16:e1008546. https://doi.org/10.1371/journal.pgen.1008546
Wu J, Xu J, Liu B, Yao G, Wang P, Lin Z, Huang B et al (2018) Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature 557:256–260. https://doi.org/10.1038/s41586-018-0080-8
Lu F, Liu Y, Inoue A, Suzuki T, Zhao K, Zhang Y (2016) Establishing chromatin regulatory landscape during mouse preimplantation development. Cell 165:1375–1388. https://doi.org/10.1016/j.cell.2016.05.050
Simandi Z, Horvath A, Wright LC, Cuaranta-Monroy I, De Luca I, Karolyi K, Sauer S et al (2016) OCT4 acts as an integrator of pluripotency and signal-induced differentiation. Mol Cell 63:647–661. https://doi.org/10.1016/j.molcel.2016.06.039
Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN (1982) The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J 1:681–686. https://doi.org/10.1002/j.1460-2075.1982.tb01230.x
Dobson AT, Raja R, Abeyta MJ, Taylor T, Shen S, Haqq C, Pera RA (2004) The unique transcriptome through day 3 of human preimplantation development. Hum Mol Genet 13:1461–1470. https://doi.org/10.1093/hmg/ddh157
Palmieri SL, Peter W, Hess H, Scholer HR (1994) Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol 166:259–267. https://doi.org/10.1006/dbio.1994.1312
Khan DR, Dube D, Gall L, Peynot N, Ruffini S, Laffont L, Le Bourhis D et al (2012) Expression of pluripotency master regulators during two key developmental transitions: EGA and early lineage specification in the bovine embryo. PLoS One 7:e34110. https://doi.org/10.1371/journal.pone.0034110
Niakan KK, Eggan K (2013) Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol 375:54–64. https://doi.org/10.1016/j.ydbio.2012.12.008
Blakeley P, Fogarty NM, Del Valle I, Wamaitha SE, Hu TX, Elder K, Snell P et al (2015) Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 142:3613. https://doi.org/10.1242/dev.131235
Gao L, Wu K, Liu Z, Yao X, Yuan S, Tao W, Yi L et al (2018) Chromatin accessibility landscape in human early embryos and its association with evolution. Cell 173(248–259):e15. https://doi.org/10.1016/j.cell.2018.02.028
Hendrickson PG, Dorais JA, Grow EJ, Whiddon JL, Lim JW, Wike CL, Weaver BD et al (2017) Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet 49:925–934. https://doi.org/10.1038/ng.3844
De Iaco A, Planet E, Coluccio A, Verp S, Duc J, Trono D (2017) DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet 49:941–945. https://doi.org/10.1038/ng.3858
Yang F, Huang X, Zang R, Chen J, Fidalgo M, Sanchez-Priego C, Yang J et al (2020) DUX-miR-344-ZMYM2-mediated activation of MERVL LTRs induces a totipotent 2C-like state. Cell Stem Cell 26(234–250):e7. https://doi.org/10.1016/j.stem.2020.01.004
Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A et al (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487:57–63. https://doi.org/10.1038/nature11244
Eckersley-Maslin M, Alda-Catalinas C, Blotenburg M, Kreibich E, Krueger C, Reik W (2019) Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev 33:194–208. https://doi.org/10.1101/gad.321174.118
Stirparo GG, Kurowski A, Yanagida A, Bates LE, Strawbridge SE, Hladkou S, Stuart HT et al (2021) OCT4 induces embryonic pluripotency via STAT3 signaling and metabolic mechanisms. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2008890118
Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, Lea R et al (2017) Genome editing reveals a role for OCT4 in human embryogenesis. Nature 550:67–73. https://doi.org/10.1038/nature24033
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL et al (2010) The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 6:167–174. https://doi.org/10.1016/j.stem.2009.12.009
Buganim Y, Markoulaki S, van Wietmarschen N, Hoke H, Wu T, Ganz K, Akhtar-Zaidi B et al (2014) The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell 15:295–309. https://doi.org/10.1016/j.stem.2014.07.003
Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X et al (2013) Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell 12:453–469. https://doi.org/10.1016/j.stem.2013.02.005
Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H et al (2012) Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10:465–472. https://doi.org/10.1016/j.stem.2012.02.021
Kim KP, Wu Y, Yoon J, Adachi K, Wu G, Velychko S, MacCarthy CM et al (2020) Reprogramming competence of OCT factors is determined by transactivation domains. Sci Adv. https://doi.org/10.1126/sciadv.aaz7364
Kim KP, Choi J, Yoon J, Bruder JM, Shin B, Kim J, Arauzo-Bravo MJ et al (2021) Permissive epigenomes endow reprogramming competence to transcriptional regulators. Nat Chem Biol 17:47–56. https://doi.org/10.1038/s41589-020-0618-6
Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP et al (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:471–487. https://doi.org/10.1016/j.stem.2014.07.002
Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D et al (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158:1254–1269. https://doi.org/10.1016/j.cell.2014.08.029
Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D et al (2012) Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–479. https://doi.org/10.1016/j.stem.2012.03.003
Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K et al (2009) Oct4-induced pluripotency in adult neural stem cells. Cell 136:411–419. https://doi.org/10.1016/j.cell.2009.01.023
Choi HW, Kim JS, Choi S, Hong YJ, Kim MJ, Seo HG, Do JT (2014) Neural stem cells differentiated from iPS cells spontaneously regain pluripotency. Stem Cells 32:2596–2604. https://doi.org/10.1002/stem.1757
Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH (2004) Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430:350–356. https://doi.org/10.1038/nature02604
Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, Lendahl U et al (2000) Generalized potential of adult neural stem cells. Science 288:1660–1663. https://doi.org/10.1126/science.288.5471.1660
Galli R, Borello U, Gritti A, Minasi MG, Bjornson CR, Coletta M, Mora M et al (2000) Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci 3:986–991. https://doi.org/10.1038/79924
Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL (1999) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283:534–537. https://doi.org/10.1126/science.283.5401.534
Zalc A, Sinha R, Gulati GS, Wesche DJ, Daszczuk P, Swigut T, Weissman IL et al (2021) Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science. https://doi.org/10.1126/science.abb4776
Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, Alencar GF et al (2016) Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med 22:657–665. https://doi.org/10.1038/nm.4109
Acknowledgements
We are grateful to Areti Malapetsas for editing the article and to Alexander Volkov for drawing the figures.
Funding
The work was supported by the Russian Science Foundation (RSF) Grant no. 20-74-00072 and by the Ministry of Science and Higher Education (Agreement no. 075-15-2020-773).
Author information
Authors and Affiliations
Contributions
EB collected and analyzed the data and wrote the first draft. AT corrected and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakhmet, E.I., Tomilin, A.N. Key features of the POU transcription factor Oct4 from an evolutionary perspective. Cell. Mol. Life Sci. 78, 7339–7353 (2021). https://doi.org/10.1007/s00018-021-03975-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03975-8