Abstract
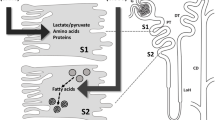
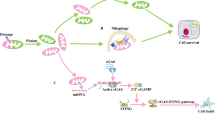
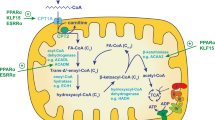
Acute kidney injury (AKI) is one of the most common clinical syndromes. AKI is associated with significant morbidity and subsequent chronic kidney disease (CKD) development. Thus, it is urgent to develop a strategy to hinder AKI progression. Renal tubules are responsible for the reabsorption and secretion of various solutes and the damage to this part of the nephron is a key mediator of AKI. As we know, many common renal insults primarily target the highly metabolically active proximal tubular cells (PTCs). PTCs are the most energy-demanding cells in the kidney. The ATP that they use is mostly produced in their mitochondria by fatty acid β-oxidation (FAO). But, when PTCs face various biological stresses, FAO will shut down for a time that outlives injury. Recent studies have suggested that surviving PTCs can adapt to FAO disruption by increasing glycolysis when facing metabolic constraints, although PTCs do not perform glycolysis in a normal physiological state. Enhanced glycolysis in a short period compensates for impaired energy production and exerts partial renal-protective effects, but its long-term effect on renal function and AKI progression is not promising. Deranged FAO and enhanced glycolysis may contribute to the AKI to CKD transition through different molecular biological mechanisms. In this review, we concentrate on the recent pathological findings of AKI with regards to the metabolic reprogramming in PTCs, confirming that targeting metabolic reprogramming represents a potentially effective therapeutic strategy for the progression of AKI.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, Toussaint ND, Bellomo R (2019) Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 95(1):160–172. https://doi.org/10.1016/j.kint.2018.08.036
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS (2018) Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14(10):607–625. https://doi.org/10.1038/s41581-018-0052-0
Bhargava P, Schnellmann RG (2017) Mitochondrial energetics in the kidney. Nat Rev Nephrol 13(10):629–646. https://doi.org/10.1038/nrneph.2017.107
Scholz H, Boivin FJ, Schmidt-Ott KM, Bachmann S, Eckardt KU, Scholl UI, Persson PB (2021) Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol 17(5):335–349. https://doi.org/10.1038/s41581-021-00394-7
Isaka Y, Kimura T, Takabatake Y (2011) The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells. Autophagy 7(9):1085–1087. https://doi.org/10.4161/auto.7.9.16465
Kaushal GP, Kaushal V, Herzog C, Yang C (2008) Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 4(5):710–712. https://doi.org/10.4161/auto.6309
Li M, Li CM, Ye ZC, Huang J, Li Y, Lai W, Peng H, Lou TQ (2020) Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J Cell Mol Med 24(9):5109–5121. https://doi.org/10.1111/jcmm.15148
Bataille A, Galichon P, Chelghoum N, Oumoussa BM, Ziliotis MJ, Sadia I, Vandermeersch S, Simon-Tillaux N, Legouis D, Cohen R, Xu-Dubois YC, Commereuc M, Rondeau E, Le Crom S, Hertig A (2018) Increased fatty acid oxidation in differentiated proximal tubular cells surviving a reversible episode of acute kidney injury. Cell Physiol Biochem 47(4):1338–1351. https://doi.org/10.1159/000490819
Emma F, Montini G, Parikh SM, Salviati L (2016) Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol 12(5):267–280. https://doi.org/10.1038/nrneph.2015.214
Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA (2016) Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27(11):3356–3367. https://doi.org/10.1681/asn.2015020177
Li M, Jia F, Zhou H, Di J, Yang M (2018) Elevated aerobic glycolysis in renal tubular epithelial cells influences the proliferation and differentiation of podocytes and promotes renal interstitial fibrosis. Eur Rev Med Pharmacol Sci 22(16):5082–5090. https://doi.org/10.26355/eurrev_201808_15701
Shen Y, Jiang L, Wen P, Ye Y, Zhang Y, Ding H, Luo J, Xu L, Zen K, Zhou Y, Yang J (2020) Tubule-derived lactate is required for fibroblast activation in acute kidney injury. Am J Physiol Renal Physiol 318(3):F689–F701. https://doi.org/10.1152/ajprenal.00229.2019
Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, Kaufman BA, Park J, Pei L, Baur J, Palmer M, Susztak K (2019) Mitochondrial damage and activation of the sting pathway lead to renal inflammation and fibrosis. Cell Metab 30(4):784-799.e785. https://doi.org/10.1016/j.cmet.2019.08.003
Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z (2019) Lipid accumulation and chronic kidney disease. Nutrients. https://doi.org/10.3390/nu11040722
Casals N, Zammit V, Herrero L, Fadó R, Rodríguez-Rodríguez R, Serra D (2016) Carnitine palmitoyltransferase 1C: from cognition to cancer. Prog Lipid Res 61:134–148. https://doi.org/10.1016/j.plipres.2015.11.004
Foster DW (2012) Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J Clin Invest 122(6):1958–1959. https://doi.org/10.1172/jci63967
Zuk A, Bonventre JV (2016) Acute kidney injury. Annu Rev Med 67:293–307. https://doi.org/10.1146/annurev-med-050214-013407
Swe MT, Pongchaidecha A, Chatsudthipong V, Chattipakorn N, Lungkaphin A (2019) Molecular signaling mechanisms of renal gluconeogenesis in nondiabetic and diabetic conditions. J Cell Physiol 234(6):8134–8151. https://doi.org/10.1002/jcp.27598
Sharma R, Tiwari S (2021) Renal gluconeogenesis in insulin resistance: a culprit for hyperglycemia in diabetes. World J Diabetes 12(5):556–568. https://doi.org/10.4239/wjd.v12.i5.556
Legouis D, Faivre A, Cippà PE, de Seigneux S (2020) Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa302
Uchida S, Endou H (1988) Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255(5):F977–F983. https://doi.org/10.1152/ajprenal.1988.255.5.F977 (Pt 2)
Forbes JM (2016) Mitochondria-power players in kidney function? Trends Endocrinol Metab 27(7):441–442. https://doi.org/10.1016/j.tem.2016.05.002
Sun J, Zhang J, Tian J, Virzì GM, Digvijay K, Cueto L, Yin Y, Rosner MH, Ronco C (2019) Mitochondria in sepsis-induced AKI. J Am Soc Nephrol 30(7):1151–1161. https://doi.org/10.1681/asn.2018111126
Jiang M, Bai M, Lei J, Xie Y, Xu S, Jia Z, Zhang A (2020) Mitochondrial dysfunction and the AKI-to-CKD transition. Am J Physiol Renal Physiol 319(6):F1105–F1116. https://doi.org/10.1152/ajprenal.00285.2020
Quirós PM, Mottis A, Auwerx J (2016) Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 17(4):213–226. https://doi.org/10.1038/nrm.2016.23
Chambers JM, Wingert RA (2020) PGC-1α in disease: recent renal insights into a versatile metabolic regulator. Cells. https://doi.org/10.3390/cells9102234
Cheng CF, Ku HC, Lin H (2018) PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. https://doi.org/10.3390/ijms19113447
Li SY, Susztak K (2018) The role of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in kidney disease. Semin Nephrol 38(2):121–126. https://doi.org/10.1016/j.semnephrol.2018.01.003
Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM (2011) PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121(10):4003–4014. https://doi.org/10.1172/jci58662
Rasbach KA, Schnellmann RG (2007) PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355(3):734–739. https://doi.org/10.1016/j.bbrc.2007.02.023
Wang D, Wang Y, Zou X, Shi Y, Liu Q, Huyan T, Su J, Wang Q, Zhang F, Li X, Tie L (2020) FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br J Pharmacol 177(2):432–448. https://doi.org/10.1111/bph.14878
Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM (2016) PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531(7595):528–532. https://doi.org/10.1038/nature17184
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065. https://doi.org/10.1126/science.1219855
Brooks C, Wei Q, Cho SG, Dong Z (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119(5):1275–1285. https://doi.org/10.1172/jci37829
Qin N, Cai T, Ke Q, Yuan Q, Luo J, Mao X, Jiang L, Cao H, Wen P, Zen K, Zhou Y, Yang J (2019) UCP2-dependent improvement of mitochondrial dynamics protects against acute kidney injury. J Pathol 247(3):392–405. https://doi.org/10.1002/path.5198
Gall JM, Wang Z, Liesa M, Molina A, Havasi A, Schwartz JH, Shirihai O, Borkan SC, Bonegio RG (2012) Role of mitofusin 2 in the renal stress response. PLoS ONE 7(1):e31074. https://doi.org/10.1371/journal.pone.0031074
Morigi M, Perico L, Benigni A (2018) Sirtuins in renal health and disease. J Am Soc Nephrol 29(7):1799–1809. https://doi.org/10.1681/asn.2017111218
Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A (2015) Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125(2):715–726. https://doi.org/10.1172/jci77632
Wang Q, Xu J, Li X, Liu Z, Han Y, Xu X, Li X, Tang Y, Liu Y, Yu T, Li X (2019) Sirt3 modulate renal ischemia-reperfusion injury through enhancing mitochondrial fusion and activating the ERK-OPA1 signaling pathway. J Cell Physiol 234(12):23495–23506. https://doi.org/10.1002/jcp.28918
Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH (2014) Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171(8):2017–2028. https://doi.org/10.1111/bph.12468
Zager RA, Johnson AC, Becker K (2014) Renal cortical pyruvate depletion during AKI. J Am Soc Nephrol 25(5):998–1012. https://doi.org/10.1681/asn.2013070791
Console L, Scalise M, Giangregorio N, Tonazzi A, Barile M, Indiveri C (2020) The link between the mitochondrial fatty acid oxidation derangement and kidney injury. Front Physiol 11:794. https://doi.org/10.3389/fphys.2020.00794
Varga T, Czimmerer Z, Nagy L (2011) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 1812(8):1007–1022. https://doi.org/10.1016/j.bbadis.2011.02.014
Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY (2018) Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J Am Soc Nephrol 29(4):1223–1237. https://doi.org/10.1681/asn.2017070802
Iwaki T, Bennion BG, Stenson EK, Lynn JC, Otinga C, Djukovic D, Raftery D, Fei L, Wong HR, Liles WC, Standage SW (2019) PPARα contributes to protection against metabolic and inflammatory derangements associated with acute kidney injury in experimental sepsis. Physiol Rep 7(10):e14078. https://doi.org/10.14814/phy2.14078
Freitas-Lima LC, Budu A, Arruda AC, Perilhão MS, Barrera-Chimal J, Araujo RC, Estrela GR (2020) PPAR-α deletion attenuates cisplatin nephrotoxicity by modulating renal organic transporters MATE-1 and OCT-2. Int J Mol Sci. https://doi.org/10.3390/ijms21197416
Wu SH, Chen XQ, Lü J, Wang MJ (2016) BML-111 attenuates renal ischemia/reperfusion injury via peroxisome proliferator-activated receptor-α-regulated heme oxygenase-1. Inflammation 39(2):611–624. https://doi.org/10.1007/s10753-015-0286-y
Sivarajah A, Chatterjee PK, Hattori Y, Brown PA, Stewart KN, Todorovic Z, Mota-Filipe H, Thiemermann C (2002) Agonists of peroxisome-proliferator activated receptor-alpha (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Med Sci Monit 8(12):Br532–Br539
Li S, Wu P, Yarlagadda P, Vadjunec NM, Proia AD, Harris RA, Portilla D (2004) PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286(3):F572–F580. https://doi.org/10.1152/ajprenal.00190.2003
Jang HS, Noh MR, Jung EM, Kim WY, Southekal S, Guda C, Foster KW, Oupicky D, Ferrer FA, Padanilam BJ (2020) Proximal tubule cyclophilin d regulates fatty acid oxidation in cisplatin-induced acute kidney injury. Kidney Int 97(2):327–339. https://doi.org/10.1016/j.kint.2019.08.019
Idrovo JP, Yang WL, Nicastro J, Coppa GF, Wang P (2012) Stimulation of carnitine palmitoyltransferase 1 improves renal function and attenuates tissue damage after ischemia/reperfusion. J Surg Res 177(1):157–164. https://doi.org/10.1016/j.jss.2012.05.053
Erpicum P, Rowart P, Defraigne JO, Krzesinski JM, Jouret F (2018) What we need to know about lipid-associated injury in case of renal ischemia-reperfusion. Am J Physiol Renal Physiol 315(6):F1714–F1719. https://doi.org/10.1152/ajprenal.00322.2018
Smith JA, Stallons LJ, Schnellmann RG (2014) Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am J Physiol Renal Physiol 307(4):F435–F444. https://doi.org/10.1152/ajprenal.00271.2014
Zhao X, Sun K, Lan Z, Song W, Cheng L, Chi W, Chen J, Huo Y, Xu L, Liu X, Deng H, Siegenthaler JA, Chen L (2017) Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity. Sci Rep 7:46344. https://doi.org/10.1038/srep46344
Kierans SJ, Taylor CT (2021) Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol 599(1):23–37. https://doi.org/10.1113/jp280572
Conde E, Alegre L, Blanco-Sánchez I, Sáenz-Morales D, Aguado-Fraile E, Ponte B, Ramos E, Sáiz A, Jiménez C, Ordoñez A, López-Cabrera M, del Peso L, de Landázuri MO, Liaño F, Selgas R, Sanchez-Tomero JA, García-Bermejo ML (2012) Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS ONE 7(3):e33258. https://doi.org/10.1371/journal.pone.0033258
Conde E, Giménez-Moyano S, Martín-Gómez L, Rodríguez M, Ramos ME, Aguado-Fraile E, Blanco-Sanchez I, Saiz A, García-Bermejo ML (2017) HIF-1α induction during reperfusion avoids maladaptive repair after renal ischemia/reperfusion involving miR127-3p. Sci Rep 7:41099. https://doi.org/10.1038/srep41099
Lovisa S, Fletcher-Sananikone E, Sugimoto H, Hensel J, Lahiri S, Hertig A, Taduri G, Lawson E, Dewar R, Revuelta I, Kato N, Wu CJ, Bassett RL Jr, Putluri N, Zeisberg M, Zeisberg EM, LeBleu VS, Kalluri R (2020) Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal. https://doi.org/10.1126/scisignal.aaz2597
Balzer MS, Susztak K (2020) The interdependence of renal epithelial and endothelial metabolism and cell state. Sci Signal. https://doi.org/10.1126/scisignal.abb8834
Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ (2017) Chronic kidney disease. Nat Rev Dis Primers 3:17088. https://doi.org/10.1038/nrdp.2017.88
Sato Y, Takahashi M, Yanagita M (2020) Pathophysiology of AKI to CKD progression. Semin Nephrol 40(2):206–215. https://doi.org/10.1016/j.semnephrol.2020.01.011
Harzandi A, Lee S, Bidkhori G, Saha S, Hendry BM, Mardinoglu A, Shoaie S, Sharpe CC (2021) Acute kidney injury leading to CKD is associated with a persistence of metabolic dysfunction and hypertriglyceridemia. iScience 24(2):102046. https://doi.org/10.1016/j.isci.2021.102046
Simon N, Hertig A (2015) Alteration of fatty acid oxidation in tubular epithelial cells: from acute kidney injury to renal fibrogenesis. Front Med (Lausanne) 2:52. https://doi.org/10.3389/fmed.2015.00052
Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K (2015) Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21(1):37–46. https://doi.org/10.1038/nm.3762
Lv T, Hu Y, Ma Y, Zhen J, Xin W, Wan Q (2019) GCN5L1 controls renal lipotoxicity through regulating acetylation of fatty acid oxidation enzymes. J Physiol Biochem 75(4):597–606. https://doi.org/10.1007/s13105-019-00711-6
Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL (2013) CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension 61(1):216–224. https://doi.org/10.1161/hypertensionaha.112.198770
Gao X, Wu J, Qian Y, Fu L, Wu G, Xu C, Mei C (2014) Oxidized high-density lipoprotein impairs the function of human renal proximal tubule epithelial cells through CD36. Int J Mol Med 34(2):564–572. https://doi.org/10.3892/ijmm.2014.1799
Arici M, Chana R, Lewington A, Brown J, Brunskill NJ (2003) Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J Am Soc Nephrol 14(1):17–27. https://doi.org/10.1097/01.asn.0000042167.66685.ea
Souza AC, Bocharov AV, Baranova IN, Vishnyakova TG, Huang YG, Wilkins KJ, Hu X, Street JM, Alvarez-Prats A, Mullick AE, Patterson AP, Remaley AT, Eggerman TL, Yuen PS, Star RA (2016) Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int 89(4):809–822. https://doi.org/10.1016/j.kint.2015.12.043
Yokoi H, Yanagita M (2016) Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int 89(4):740–742. https://doi.org/10.1016/j.kint.2016.01.009
Hou Y, Wang Q, Han B, Chen Y, Qiao X, Wang L (2021) CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis 12(6):523. https://doi.org/10.1038/s41419-021-03813-6
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O’Neill LA (2015) Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 21(1):65–80. https://doi.org/10.1016/j.cmet.2014.12.005
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496(7444):238–242. https://doi.org/10.1038/nature11986
Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, Kang R, Lotze MT, Billiar TR, Wang H, Cao L, Tang D (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 5:4436. https://doi.org/10.1038/ncomms5436
Li L, Tang L, Yang X, Chen R, Zhang Z, Leng Y, Chen AF (2020) Gene regulatory effect of pyruvate kinase M2 is involved in renal inflammation in type 2 diabetic nephropathy. Exp Clin Endocrinol Diabetes 128(9):599–606. https://doi.org/10.1055/a-1069-7290
Brown TP, Ganapathy V (2020) Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther 206:107451. https://doi.org/10.1016/j.pharmthera.2019.107451
San-Millán I, Brooks GA (2017) Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg effect. Carcinogenesis 38(2):119–133. https://doi.org/10.1093/carcin/bgw127
Hirschhaeuser F, Sattler UG, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71(22):6921–6925. https://doi.org/10.1158/0008-5472.can-11-1457
Britland S, Ross-Smith O, Jamil H, Smith AG, Vowden K, Vowden P (2012) The lactate conundrum in wound healing: clinical and experimental findings indicate the requirement for a rapid point-of-care diagnostic. Biotechnol Prog 28(4):917–924. https://doi.org/10.1002/btpr.1561
Cui H, Xie N, Banerjee S, Ge J, Jiang D, Dey T, Matthews QL, Liu RM, Liu G (2021) Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am J Respir Cell Mol Biol 64(1):115–125. https://doi.org/10.1165/rcmb.2020-0360OC
Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G (2015) Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192(12):1462–1474. https://doi.org/10.1164/rccm.201504-0780OC
Rutz HP, Little JB (1995) Exogenous lactate interferes with cell-cycle control in Balb/3T3 mouse fibroblasts. Int J Radiat Oncol Biol Phys 31(3):525–528. https://doi.org/10.1016/0360-3016(94)00362-o
Wagner S, Hussain MZ, Beckert S, Ghani QP, Weinreich J, Hunt TK, Becker HD, Königsrainer A (2007) Lactate down-regulates cellular poly(ADP-ribose) formation in cultured human skin fibroblasts. Eur J Clin Invest 37(2):134–139. https://doi.org/10.1111/j.1365-2362.2007.01760.x
Takahashi K, Kamijo Y, Hora K, Hashimoto K, Higuchi M, Nakajima T, Ehara T, Shigematsu H, Gonzalez FJ, Aoyama T (2011) Pretreatment by low-dose fibrates protects against acute free fatty acid-induced renal tubule toxicity by counteracting PPARα deterioration. Toxicol Appl Pharmacol 252(3):237–249. https://doi.org/10.1016/j.taap.2011.02.012
Kaur J, Kaur T, Sharma AK, Kaur J, Yadav HN, Pathak D, Singh AP (2021) Fenofibrate attenuates ischemia reperfusion-induced acute kidney injury and associated liver dysfunction in rats. Drug Dev Res 82(3):412–421. https://doi.org/10.1002/ddr.21764
Li L, Emmett N, Mann D, Zhao X (2010) Fenofibrate attenuates tubulointerstitial fibrosis and inflammation through suppression of nuclear factor-κB and transforming growth factor-β1/Smad3 in diabetic nephropathy. Exp Biol Med (Maywood) 235(3):383–391. https://doi.org/10.1258/ebm.2009.009218
Balakumar P, Sambathkumar R, Mahadevan N, Muhsinah AB, Alsayari A, Venkateswaramurthy N, Dhanaraj SA (2019) Molecular targets of fenofibrate in the cardiovascular-renal axis: a unifying perspective of its pleiotropic benefits. Pharmacol Res 144:132–141. https://doi.org/10.1016/j.phrs.2019.03.025
Emami F, Hariri A, Matinfar M, Nematbakhsh M (2020) Fenofibrate-induced renal dysfunction, yes or no? J Res Med Sci 25:39. https://doi.org/10.4103/jrms.JRMS_772_19
Gu D, Fang D, Zhang M, Guo J, Ren H, Li X, Zhang Z, Yang D, Zou X, Liu Y, Wang WE, Wu G, Jose PA, Han Y, Zeng C (2021) Gastrin, via activation of PPARα, protects the kidney against hypertensive injury. Clin Sci (Lond) 135(2):409–427. https://doi.org/10.1042/cs20201340
Hao Y, Miao J, Liu W, Peng L, Chen Y, Zhong Q (2021) Formononetin protects against cisplatin-induced acute kidney injury through activation of the PPARα/Nrf2/HO-1/NQO1 pathway. Int J Mol Med 47(2):511–522. https://doi.org/10.3892/ijmm.2020.4805
Szeto HH (2017) Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol 28(10):2856–2865. https://doi.org/10.1681/asn.2017030247
Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB (2009) The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20(11):2380–2388. https://doi.org/10.1681/asn.2008111138
Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG (2012) The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342(1):106–118. https://doi.org/10.1124/jpet.112.191528
Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG (2014) Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25(6):1157–1162. https://doi.org/10.1681/asn.2013090952
Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, Feldman LC, Gustafsson T (2017) Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol 28(5):1437–1449. https://doi.org/10.1681/asn.2016070761
Liu S, Soong Y, Seshan SV, Szeto HH (2014) Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol 306(9):F970–F980. https://doi.org/10.1152/ajprenal.00697.2013
Szeto HH, Liu S, Soong Y, Birk AV (2015) Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol 308(1):F11–F21. https://doi.org/10.1152/ajprenal.00366.2014
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZL, SL and XL all reviewed the literature and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Lu, S. & Li, X. The role of metabolic reprogramming in tubular epithelial cells during the progression of acute kidney injury. Cell. Mol. Life Sci. 78, 5731–5741 (2021). https://doi.org/10.1007/s00018-021-03892-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03892-w