Abstract
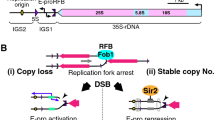
Saccharomyces cerevisiae ribosomal DNA, the repeated region where rRNAs are synthesized by about 150 encoding units, hosts all the protein machineries responsible for the main DNA transactions such as replication, transcription and recombination. This and its repetitive nature make rDNA a unique and complex genetic locus compared to any other. All the different molecular machineries acting in this locus need to be accurately and finely controlled and coordinated and for this reason rDNA is one of the most impressive examples of highly complex molecular regulated loci. The region in which the large molecular complexes involved in rDNA activity and/or regulation are recruited is extremely small: that is, the 2.5 kb long intergenic spacer, interrupting each 35S RNA coding unit from the next. All S. cerevisiae RNA polymerases (I, II and III) transcribing the different genetic rDNA elements are recruited here; a sequence responsible for each rDNA unit replication, which needs its molecular apparatus, also localizes here; moreover, it is noteworthy that the rDNA replication proceeds almost unidirectionally because each replication fork is stopped in the so-called replication fork barrier. These localized fork blocking events induce, with a given frequency, the homologous recombination process by which cells maintain a high identity among the rDNA repeated units. Here, we describe the different processes involving the rDNA locus, how they influence each other and how these mutual interferences are highly regulated and coordinated. We propose that an rDNA conformation as a super-hub could help in optimizing the micro-environment for all basic DNA transactions.





Similar content being viewed by others
References
Petes TD (1979) Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci USA 76:410–414
Tsai A, Alves MR, Crocker J (2019) Multi-enhancer transcriptional hubs confer phenotypic robustness. eLife 8:e45325. https://doi.org/10.7554/eLife.45325
Skryabin KG, Eldarov MA, Larionov VL, Bayev AA, Klootwijk J, de Regt VC, Veldman GM, Planta RJ, Georgiev OI, Hadjiolov AA (1984) Structure and function of the nontranscribed spacer regions of yeast rDNA. Nucleic Acids Res 12:2955–2968
Ganley AR, Hayashi K, Horiuchi T, Kobayashi T (2005) Identifying gene-independent noncoding functional elements in the yeast ribosomal DNA by phylogenetic footprinting. Proc Natl Acad Sci US Am 102(33):11787–11792
Mayan M, Aragon L (2010) Cis-interactions between non-coding ribosomal spacers dependent on RNAP-II separate RNAP-I and RNAP-III transcription domains. Cell Cycle 9:4328–4337. https://doi.org/10.4161/cc.9.21.13591
Smith JS, Boeke JD (1997) An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev 11:241–254. https://doi.org/10.1101/gad.11.2.241
Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ (1997) Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev 11:255–269. https://doi.org/10.1101/gad.11.2.255
Brewer BJ, Fangman WL (1988) A replication fork barrier at the 3’ end of yeast ribosomal RNA genes. Cell 55:637–643
Kobayashi T, Hidaka M, Nishizawa M, Horiuchi T (1992) Identification of a site required for DNA replication fork blocking activity in the rRNA gene cluster in Saccharomyces cerevisiae. Mol Gen Genet 233:355–362. https://doi.org/10.1007/bf00265431
Kobayashi T, Horiuchi T (1996) A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells 1:465–474
Kobayashi T (2011) Regulation of ribosomal RNA gene copy number and its role in modulating genome integrity and evolutionary adaptability in yeast. Cell Mol Life Sci 68:1395–1403. https://doi.org/10.1007/s00018-010-0613-2
Kulkens T, Riggs DL, Heck JD, Planta RJ, Nomura M (1991) The yeast RNA polymerase I promoter: ribosomal DNA sequences involved in transcription initiation and complex formation in vitro. Nucleic Acids Res 19:5363–5370
Bordi L, Cioci F, Camilloni G (2001) In vivo binding and hierarchy of assembly of the yeast RNA polymerase I transcription factors. Mol Biol Cell 12:753–760. https://doi.org/10.1091/mbc.12.3.753
Yamamoto RT, Nogi Y, Dodd JA, Nomura M (1996) RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J 15:3964–3973
Keys DA, Lee BS, Dodd JA, Nguyen TT, Vu L, Fantino E, Burson LM, Nogi Y, Nomura M (1996) Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev 10:887–903
Lalo D, Steffan JS, Dodd JA, Nomura M (1996) RRN11 encodes the third subunit of the complex containing Rrn6p and Rrn7p that is essential for the initiation of rDNA transcription by yeast RNA polymerase I. J Biol Chem 271:21062–21067
Steffan JS, Keys DA, Dodd JA, Nomura M (1996) The role of TBP in rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae: TBP is required for upstream activation factor-dependent recruitment of core factor. Genes Dev 10(2551–2563):12
Vogelauer M, Camilloni G (1999) Site-specific in vivo cleavages by DNA topoisomerase I in the regulatory regions of the 35 S rRNA in Saccharomyces cerevisiae are transcription independent. J Mol Biol 293:19–28. https://doi.org/10.1006/jmbi.1999.3154
Bonven BJ, Gocke E, Westergaard O (1985) A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell 41:541–551. https://doi.org/10.1016/s0092-8674(85)80027-1
Vogelauer M, Cioci F, Camilloni G (1998) DNA protein-interactions at the Saccharomyces cerevisiae 35 S rRNA promoter and in its surrounding region. J Mol Biol 275:197–209. https://doi.org/10.1006/jmbi.1997.1451
Li C, Mueller JE, Bryk M (2006) Sir2 represses endogenous polymerase II transcription units in the ribosomal DNA nontranscribed spacer. Mol Biol Cell 17:3848–3859
Laloraya S, Guacci V, Koshland D (2000) Chromosomal addresses of the cohesin component Mcd1p. J Cell Biol 151:1047–1056. https://doi.org/10.1083/jcb.151.5.1047
Houseley J, Kotovic K, El Hage A, Tollervey D (2007) Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J 26:4996–5006. https://doi.org/10.1038/sj.emboj.7601921
Felice FD, Cioci F, Camilloni G (2005) FOB1 affects DNA topoisomerase I in vivo cleavages in the enhancer region of the Saccharomyces cerevisiae ribosomal DNA locus. Nucl Acids Res 33:6327–6337. https://doi.org/10.1093/nar/gki950
Choudhury M, Zaman S, Jiang JC, Jazwinski SM, Bastia D (2015) Mechanism of regulation of ‘chromosome kissing’ induced by Fob1 and its physiological significance. Genes Dev 29:1188–1201. https://doi.org/10.1101/gad.260844.115
Lang WH, Reeder RH (1993) The REB1 site is an essential component of a terminator for RNA polymerase I in Saccharomyces cerevisiae. Mol Cell Biol 13:649–658. https://doi.org/10.1128/mcb.13.1.649
Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA, Noble WS (2010) A three-dimensional model of the yeast genome. Nature 465:363–367. https://doi.org/10.1038/nature08973
Lin CW, Moorefield B, Payne J, Aprikian P, Mitomo K, Reeder RH (1996) A novel 66- kilodalton protein complexes with Rrn6, Rrn7, and TATA-binding protein to promote polymerase I transcription initiation in Saccharomyces cerevisiae. Mol Cell Biol 16:6436–6443. https://doi.org/10.1128/mcb.16.11.6436
Geiduschek EP, Kassavetis GA (2001) The RNA polymerase III transcription apparatus. J Mol Biol 310:1–26. https://doi.org/10.1006/jmbi.2001.4732
Conrad-Webb H, Butow RA (1995) A polymerase switch in the synthesis of rRNA in Saccharomyces cerevisiae. Mol Cell Biol 15:2420–2428. https://doi.org/10.1128/mcb.15.5.2420
Vu L, Siddiqi I, Lee B-S, Josaitis CA, Nomura M (1999) RNA polymerase switch in transcription of yeast rDNA: role of transcription factor UAF (upstream activation factor) in silencing rDNA transcription by RNA polymerase II. Proc Natl Acad Sci 96(4390–4395):13
Cesarini E, Mariotti FR, Cioci F, Camilloni G (2010) RNA polymerase I transcription silences noncoding RNAs at the ribosomal DNA locus in Saccharomyces cerevisiae. Eukaryot Cell 9:325–335. https://doi.org/10.1128/EC.00280-09
Mohanty BK, Bairwa NK, Bastia D (2006) The Tof1p–Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. PNAS 103:897–902. https://doi.org/10.1073/pnas.0506540103
Dammann R, Lucchini R, Koller T, Sogo JM (1995) Transcription in the yeast rRNA gene locus: distribution of the active gene copies and chromatin structure of their flanking regulatory sequences. Mol Cell Biol 15:5294–5303. https://doi.org/10.1128/mcb.15.10.5294
Fangman WL, Brewer BJ (1991) Activation of replication origins within yeast chromosomes. Annu Rev Cell Biol 7:375–402. https://doi.org/10.1146/annurev.cb.07.110191.002111
Pasero P, Bensimon A, Schwob E (2002) Single-molecule analysis reveals clustering and epigenetic regulation of replication origins at the yeast rDNA locus. Genes Dev 16:2479–2484. https://doi.org/10.1101/gad.232902
Muller M, Lucchini R, Sogo JM (2000) Replication of yeast rDNA initiates downstream of transcriptionally active genes. Mol Cell 5:767–777. https://doi.org/10.1016/s1097-2765(00)80317-2
Shyian M, Mattarocci S, Albert B, Hafner L, Lezaja A, Costanzo M, Boone C, Shore D (2016) Budding yeast Rif1 controls genome integrity by inhibiting rDNA replication. PLoS Genet 12(11):e1006414. https://doi.org/10.1371/journal.pgen.1006414
Weitao T, Budd M, Hoopes LLM, Campbell JL (2003) Dna2 helicase/nuclease causes replicative fork stalling and double-strand breaks in the ribosomal DNA of Saccharomyces cerevisiae. J Biol Chem 278:22513–22522. https://doi.org/10.1074/jbc.M301610200
Kobayashi T, Ganley ARD (2005) Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309:1581–1584. https://doi.org/10.1126/science.1116102
Smith JS, Caputo E, Boeke JD (1999) A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol Cell Biol 19:3184–3197
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800. https://doi.org/10.1038/35001622
Dutnall RN, Pillus L (2001) Deciphering NAD-dependent deacetylases. Cell 105:161–164. https://doi.org/10.1016/s0092-8674(01)00305-1
Holmes SG, Rose AB, Steuerle K, Saez E, Sayegh S, Lee YM, Broach JR (1997) Hyperactivation of the silencing proteins, Sir2p and Sir3p, causes chromosome loss. Genetics 145(605–614):14
Foss EJ, Lao U, Dalrymple E, Adrianse RL, Loe T, Bedalov A (2017) SIR2 suppresses replication gaps and genome instability by balancing replication between repetitive and unique sequences. PNAS 114:552–557. https://doi.org/10.1073/pnas.1614781114
Foss EJ, Gatbonton-Schwager T, Thiesen AH, Taylor E, Soriano R, Lao U, MacAlpine DM, Bedalov A (2019) Sir2 suppresses transcription-mediated displacement of Mcm2-7 replicative helicases at the ribosomal DNA repeats. PLoS Genet 15:e1008138. https://doi.org/10.1371/journal.pgen.1008138
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13:2570–2580. https://doi.org/10.1101/gad.13.19.2570
Mills KD, Sinclair DA, Guarente L (1999) MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97:609–620. https://doi.org/10.1016/s0092-8674(00)80772-2
Rusche LN, Kirchmaier AL, Rine J (2003) The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Ann Rev Biochem 72:481–516. https://doi.org/10.1146/annurev.biochem.72.121801.161547
Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZW, Jang J, Shevchenko A, Charbonneau H, Deshaies RJ (1999) Exit from mitosis is triggered by Tem1- dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97:233–244. https://doi.org/10.1016/s0092-8674(00)80733-3
Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M (1995) Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80:583–592. https://doi.org/10.1016/0092-8674(95)90512-x
Cioci F, Vogelauer M, Camilloni G (2002) Acetylation and accessibility of rDNA chromatin in Saccharomyces cerevisiae in Δ top1 and Δ sir2 mutants. J Mol Biol 322:41–52. https://doi.org/10.1016/S0022-2836(02)00749-0
Gottlieb S, Esposito RE (1989) A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56:771–776. https://doi.org/10.1016/0092-8674(89)90681-8
Vasiljeva L, Kim M, Terzi N, Soares LM, Buratowski S (2008) Transcription termination and RNA degradation contribute to silencing of RNA polymerase II transcription within heterochromatin. Mol Cell 29:313–323. https://doi.org/10.1016/j.molcel.2008.01.011
Kobayashi T, Horiuchi T, Tongaonkar P, Vu L, Nomura M (2004) SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117:441–453. https://doi.org/10.1016/s0092-8674(04)00414-3
Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, Itoh T, Watanabe Y, Shirahige K, Uhlmann F (2004) Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature 430:573–578. https://doi.org/10.1038/nature02742
Bausch C, Noone S, Henry JM, Gaudenz K, Sanderson B, Seidel C, Gerton JL (2007) Transcription alters chromosomal locations of cohesin in Saccharomyces cerevisiae. Mol Cell Biol 27:8522–8532. https://doi.org/10.1128/MCB.01007-0715
Azvolinsky A, Giresi PG, Lieb JD, Zakian VA (2009) Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol Cell 34:722–734. https://doi.org/10.1016/j.molcel.2009.05.022
Christman MF, Dietrich FS, Fink GR (1988) Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell 55:413–425. https://doi.org/10.1016/0092-8674(88)90027-x
Christman MF, Dietrich FS, Levin NA, Sadoff BU, Fink GR (1993) The rRNA-encoding DNA array has an altered structure in topoisomerase I mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci 90:7637–7641
D’Alfonso A, Di Felice F, Carlini V, Wright CM, Hertz MI, Bjornsti M-A, Camilloni G (2016) Molecular mechanism of DNA topoisomerase I-dependent rDNA silencing: Sir2p recruitment at ribosomal genes. J Mol Biol 428:4905–4916. https://doi.org/10.1016/j.jmb.2016.10.032
Di Felice F, Egidi A, D’Alfonso A, Camilloni G (2019) Fob1p recruits DNA topoisomerase I to ribosomal genes locus and contributes to its transcriptional silencing maintenance. Int J Biochem Cell Biol 110:143–148. https://doi.org/10.1016/j.biocel.2019.03.006
Kobayashi T, Heck DJ, Nomura M, Horiuchi T (1998) Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev 12:3821–3830. https://doi.org/10.1101/gad.12.24.3821
Defossez PA, Prusty R, Kaeberlein M, Lin SJ, Ferrigno P, Silver PA, Keil RL, Guarente L (1999) Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol Cell 3:447–455. https://doi.org/10.1016/s1097-2765(00)80472-4
Iarovaia OV, Minina EP, Sheval EV, Onichtchouk D, Dokudovskaya S, Razin SV, Vassetzky YS (2019) Nucleolus: a central hub for nuclear functions. Trends Cell Biol 29(8):647–659. https://doi.org/10.1016/j.tcb.2019.04.003
Weeks SE, Metge BJ, Samant RS (2019) The nucleolus: a central response hub for the stressors that drive cancer progression. Cell Mol Life Sci 76(22):4511–4524. https://doi.org/10.1007/s00018-019-03231-0
Moss T, Stefanovsky VY (2002) At the center of eukaryotic life. Cell 109:545–548. https://doi.org/10.1016/s0092-8674(02)00761-4
Acknowledgements
This research was supported by “Progetti di Ateneo” Universita di Roma “La Sapienza”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Egidi, A., Di Felice, F. & Camilloni, G. Saccharomyces cerevisiae rDNA as super-hub: the region where replication, transcription and recombination meet. Cell. Mol. Life Sci. 77, 4787–4798 (2020). https://doi.org/10.1007/s00018-020-03562-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03562-3