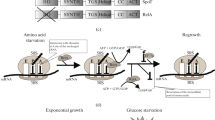
Abstract
In nature, bacteria are constantly adapting to various stressful conditions. Timely activation of stress response programs is crucial for bacteria to smoothly survive under stressful conditions. Stress response, demanding the de novo synthesis of many defense proteins, is generally activated at the transcriptional level by specific regulators. However, the effect of the global protein translational status on stress response has been largely overlooked. The translational capacity is limited by the number of translating ribosomes and the translational elongation rate. Recent work has shown that certain environmental stressors (e.g. oxidative stress) could severely compromise the stress response progress of bacteria by causing either slow-down or even complete stalling of the translational elongation process. The maintenance of ribosome elongation rate, being crucial for timely synthesis of stress defense proteins, becomes the physiological bottleneck that limits the survival of bacteria in some stressful conditions. Here, we briefly summarize some recent progress on the translational status of bacteria under two distinct stress conditions, nutrient deprivation and oxidative stress. We further discuss several important open questions on the translational regulation of bacteria during stress. The ribosome translation should be investigated in parallel with traditional transcriptional regulation in order to gain a better understanding on bacterial stress defense.

Similar content being viewed by others
References
Ron EZ (2006) Bacterial stress response. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The pro-karyotes. Springer, New York, pp 1012–1027
Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213
Henggearonis R (1993) Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72(2):165–168
Hengge-Aronis R (1993) The Role of rpoS in early stationary-phase gene regulation in Escherichia coli K12. In: Kjelleberg S (ed) Starvation in bacteria. Springer, Boston, pp 171–200
Guisbert E, Yura T, Rhodius VA, Gross CA (2008) Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev 72(3):545–554
Cornforth DM, Foster KR (2013) Competition sensing: the social side of bacterial stress responses. Nat Rev Microbiol 11(4):285–293
Christman MF, Morgan RW, Jacobson FS, Ames BN (1985) Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41(3):753–762
Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B (1990) Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA 87(16):6181–6185
Ezraty B, Gennaris A, Barras F, Collet JF (2017) Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15(7):385
Imlay JA (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11(7):443–454
Imlay JA (2015) Transcription factors that defend bacteria against reactive oxygen species. Annu Rev Microbiol 69(1):93–108
Moll I, Engelberg-Kulka H (2012) Selective translation during stress in Escherichia coli. Trends Biochem Sci 37(11):493–498
Reeve CA, Amy PS, Matin A (1984) Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol 160(3):1041–1046
Klumpp S, Scott M, Pedersen S, Hwa T (2013) Molecular crowding limits translation and cell growth. Proc Natl Acad Sci USA 110(42):16754–16759
Basan M et al (2015) Inflating bacterial cells by increased protein synthesis. Mol Syst Biol 11(10):836
Zhu M, Dai X (2018) On the intrinsic constraint of bacterial growth rate: M. tuberculosis’s view of the protein translation capacity. Crit Rev Microbiol 44(4):455–464
Bremer H, Dennis PP (1996) Modulation of chemical composition and other parameters of the cell at different exponential growth rates. In: Neidhardt FC (ed) Escherichia coli and Salmonella, 2nd edn. American Society for Microbiology, Washington, pp 1553–1569
Dai X et al (2018) Slowdown of translational elongation in Escherichia coli under hyperosmotic stress. mBio 9(1):02375-18
Dai X et al (2016) Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol 2:16231
Li SH et al (2018) Escherichia coli translation strategies differ across carbon, nitrogen and phosphorus limitation conditions. Nat Microbiol 3(8):939–947
Iyer S, Le D, Park BR, Kim M (2018) Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat Microbiol 3(6):741
Vogel U, Sorensen M, Pedersen S, Jensen KF, Kilstrup M (1992) Decreasing transcription elongation rate in Escherichia coli exposed to amino acid starvation. Mol Microbiol 6(15):2191–2200
Zhu M, Dai X (2019) Maintenance of translational elongation rate underlies the survival of Escherichia coli during oxidative stress. Nucleic Acids Res gkz467 47(14):7592–7604
Zhu M, Dai X, Wang YP (2016) Real time determination of bacterial in vivo ribosome translation elongation speed based on LacZα complementation system. Nucleic Acids Res 44(20):e155
Kolter R, Siegele DA, Tormo A (1993) The stationary phase of bacteiral life cycles. Annu Rev Microbiol 47(1):855–874
Nyström T (2004) Stationary-phase physiology. Annu Rev Microbiol 58(1):161–181
Nystrom T, Flardh K, Kjelleberg S (1990) Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol 172(12):7085–7097
Svenningsen SL, Kongstad M, Stenum TS, Muñoz-Gómez AJ, Sørensen MA (2017) Transfer RNA is highly unstable during early amino acid starvation in Escherichia coli. Nucleic Acids Res 45(2):793
Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51
Prossliner T, Skovbo Winther K, Sorensen MA, Gerdes K (2018) Ribosome Hibernation. Annu Rev Genet 52:321–348
Baracchini E, Bremer H (1988) Stringent and growth control of rRNA synthesis in Escherichia coli are both mediated by ppGpp. J Biol Chem 263(6):2597–2602
Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K (2015) Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13(5):298–309
Zhu M, Dai X (2019) Growth suppression by altered (p)ppGpp level results from non-optimal resource allocation in Escherichia coli. Nucleic Acids Res gkz211 47(9):4684–4693
Zhu M, Pan Y, Dai X (2019) (p)ppGpp: the magic governor of bacterial growth economy. Curr Genet 65(5):1121–1125
Wada A (1998) Growth phase coupled modulation of Escherichia coli ribosomes. Genes Cells 3(4):203–208
Milon P et al (2006) The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc Natl Acad Sci USA 103(38):13962–13967
Mori M, Schink S, Erickson DW, Gerland U, Hwa T (2017) Quantifying the benefit of a proteome reserve in fluctuating environments. Nat Commun 8(1):1225
Aiso T, Yoshida H, Wada A, Ohki R (2005) Modulation of mRNA stability participates in stationary-phase-specific expression of ribosome modulation factor. J Bacteriol 187(6):1951–1958
Burton NA et al (2014) Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host Microbe 15(1):72–83
Zheng M et al (2001) DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183(15):4562–4570
Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS (2009) Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324(5924):218–223
Brar GA, Weissman JS (2015) Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol 16(11):651–664
Ingolia NT (2014) Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet 15(3):205–213
Li GW, Burkhardt D, Gross C, Weissman JS (2014) Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157(3):624–635
Li GW (2015) How do bacteria tune translation efficiency? Curr Opin Microbiol 24:66–71
Pedersen S (1984) Escherichia coli ribosomes translate in vivo with variable rate. EMBO J 3(12):2895–2898
Sorensen MA, Kurland CG, Pedersen S (1989) Codon usage determines translation rate in Escherichia coli. J Mol Biol 207(2):365–377
Sorensen MA, Pedersen S (1991) Absolute in vivo translation rates of individual codons in Escherichia coli. The two glutamic acid codons GAA and GAG are translated with a threefold difference in rate. J Mol Biol 222(2):265–280
Li GW, Oh E, Weissman JS (2012) The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 484(7395):538–541
Gerashchenko MV, Lobanov AV, Gladyshev VN (2012) Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc Natl Acad Sci USA 109(43):17394–17399
Liu B, Han Y, Qian SB (2013) Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol Cell 49(3):453–463
Shalgi R et al (2013) Widespread regulation of translation by elongation pausing in heat shock. Mol Cell 49(3):439–452
Proshkin S, Rahmouni AR, Mironov A, Nudler E (2010) Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 328(5977):504–508
Newton WA, Beckwith JR, Zipser D, Brenner S (1965) Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol 14(1):290–296
Adhya S, Gottesman M (1978) Control of transcription termination. Annu Rev Biochem 47:967–996
Elgamal S, Artsimovitch I, Ibba M (2016) Maintenance of transcription–translation coupling by elongation factor P. mBio 7(5):e01373-16
Zhu M, Mori M, Hwa T, Dai X (2019) Disruption of transcription-translation coordination in Escherichia coli leads to premature transcriptional termination. Nat Microbiol. https://doi.org/10.1038/s41564-019-0543-1
Iyer S, Le D, Park BR, Kim M (2018) Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat Microbiol 3(6):741
Vogel U, Jensen KF (1994) Effects of guanosine 3′,5′-bisdiphosphate (ppGpp) on rate of transcription elongation in isoleucine-starved Escherichia coli. J Biol Chem 269(23):16236–16241
Davies KJ (2010) Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50(4–5):279–289
Nawrot B, Sochacka E, Düchler M (2011) tRNA structural and functional changes induced by oxidative stress. Cell Mol Life Sci 68(24):4023–4032
Acknowledgements
This work was supported by the National Natural Science Fund of China (No. 31700089, No. 31970027, No. 31700039 and No. 31870028) and by self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (CCNU18QN028, CCNU18KFY01, CCNU19TS028 and CCNU18ZDPY05).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, M., Dai, X. Bacterial stress defense: the crucial role of ribosome speed. Cell. Mol. Life Sci. 77, 853–858 (2020). https://doi.org/10.1007/s00018-019-03304-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03304-0