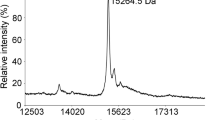
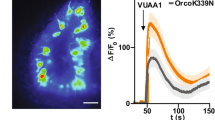
Abstract
Odorant-binding proteins (OBPs) are small soluble proteins that are thought to transport hydrophobic odorants across the aqueous sensillar lymph to olfactory receptors. A recent study revealed that OBP28a, one of the most abundant Drosophila OBPs, is not required for odorant transport, but acts in buffering rapid odour variation in the odorant environment. To further unravel and decipher its functional role, we expressed recombinant OBP28a and characterized its binding specificity. Using a fluorescent binding assay, we found that OBP28a binds a restricted number of floral-like chemicals, including ß-ionone, with an affinity in the micromolar range. We solved the X-ray crystal structure of OBP28a, which showed extensive conformation changes upon ligand binding. Mutant flies genetically deleted for the OBP28a gene showed altered responses to ß-ionone at a given concentration range, supporting its essential role in the detection of specific compounds present in the natural environment of the fly.





Similar content being viewed by others
Abbreviations
- OBPs:
-
Odorant-binding proteins
- OSNs:
-
Olfactory-sensory neurons
- Ors:
-
Odorant receptors
- cVA:
-
Cis-vaccenyl acetate
- RPLC:
-
Reverse-phase liquid chromatography
- CD:
-
Circular dichroism
- SEC:
-
Size-exclusion chromatography
- MALS:
-
Multi-angle laser light scattering
- 1PE:
-
Pentaethylene glycol
- NPN:
-
N-phenyl-1-naphthylamine
- SSR:
-
Single-sensillum recording
- lmadPBP:
-
Pheromone-binding protein from Leucophaea maderae
- dmelOBP76a:
-
Odorant-binding protein 76a from D. melanogaster
- amelASP1:
-
Pheromone-binding protein 1 from Apis mellifera
- amelOBP14:
-
Odorant-binding protein 14 from Apis mellifera
- bmorPBP:
-
Pheromone-binding protein from Bombyx mori
- agamOBP1:
-
Odorant-binding protein 1 from Anopheles gambiae
- cquiOBP1:
-
Odorant-binding protein 1 from Culex pipiens quinquefasciatus
- BMGY:
-
Buffered minimal glycerol
- YNB:
-
Yeast nitrogen base
- BMM:
-
Buffered minimal methanol
References
de Bruyne M, Foster K, Carlson JR (2001) Odor coding in the Drosophila antenna. Neuron 30:537–552. https://doi.org/10.1016/S0896-6273(01)00289-6
Ai M, Min S, Grosjean Y et al (2010) Acid sensing by the Drosophila olfactory system. Nature 468:691–695. https://doi.org/10.1038/nature09537
Yao CA, Ignell R, Carlson JR (2005) Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci 25:8359–8367. https://doi.org/10.1523/JNEUROSCI.2432-05.2005
Clyne P, Grant A, O’Connell R, Carlson JR (1997) Odorant response of individual sensilla on the Drosophila antenna. Invertebr Neurosci 3:127–135. https://doi.org/10.1007/BF02480367
Dweck HKM, Ebrahim SAM, Thoma M et al (2015) Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci 112:E2829–E2835. https://doi.org/10.1073/pnas.1504527112
Younus F, Fraser NJ, Coppin CW et al (2017) Molecular basis for the behavioral effects of the odorant degrading enzyme Esterase 6 in Drosophila. Sci Rep 7:1–12. https://doi.org/10.1038/srep46188
Larsson MC, Domingos AI, Jones WD et al (2004) Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43:703–714
Benton R, Sachse S, Michnick SW, Vosshall LB (2006) Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4:240–257. https://doi.org/10.1109/ICARCV.2014.7064338
Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136:149–162. https://doi.org/10.1016/j.cell.2008.12.001
Hussain A, Zhang M, Üçpunar HK et al (2016) Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol 14:e1002454. https://doi.org/10.1371/journal.pbio.1002454
Galindo K, Smith DP (2001) A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics 159:1059–1072
Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA (2002) Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res 12:1357–1369. https://doi.org/10.1101/gr.239402.2001
Vieira FG, Rozas J (2011) Comparative genomics of the odorant-binding and chemosensory protein gene families across the arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol 3:476–490. https://doi.org/10.1093/gbe/evr033
Anholt RRH, Williams TI (2010) The soluble proteome of the Drosophila antenna. Chem Senses 35:21–30. https://doi.org/10.1093/chemse/bjp073
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58:373–391. https://doi.org/10.1146/annurev-ento-120811-153635
Pelosi P, Iovinella I, Zhu J et al (2018) Beyond chemoreception: diverse tasks of soluble olfactory proteins in insects. Biol Rev 93:184–200. https://doi.org/10.1111/brv.12339
Pelosi P, Zhou JJ, Ban LP, Calvello M (2006) Soluble proteins in insect chemical communication. Cell Mol Life Sci 63:1658–1676. https://doi.org/10.1007/s00018-005-5607-0
Wu Z, Lin J, Zhang H, Zeng X (2016) BdorOBP83a-2 mediates responses of the oriental fruit fly to semiochemicals. Front Physiol 7:1–15. https://doi.org/10.3389/fphys.2016.00452
Jeong YT, Shim J, Oh SR et al (2013) An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron 79:725–737. https://doi.org/10.1016/j.neuron.2013.06.025
Horst R, Damberger F, Luginbühl P et al (2001) NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc Natl Acad Sci 98:14374–14379. https://doi.org/10.1073/pnas.251532998
Kruse SW, Zhao R, Smith DP, Jones DNM (2003) Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster. Nat Struct Biol 10:694–700. https://doi.org/10.1038/nsb960
Lartigue A, Gruez A, Briand L et al (2004) Sulfur single-wavelength anomalous diffraction crystal structure of a pheromone-binding protein from the honeybee Apis mellifera L. J Biol Chem 279:4459–4464. https://doi.org/10.1074/jbc.M311212200
Wogulis M, Morgan T, Ishida Y et al (2006) The crystal structure of an odorant binding protein from Anopheles gambiae: evidence for a common ligand release mechanism. Biochem Biophys Res Commun 339:157–164. https://doi.org/10.1016/j.bbrc.2005.10.191
Lescop E, Briand L, Pernollet JC, Guittet E (2009) Structural basis of the broad specificity of a general odorant-binding protein from honeybee. Biochemistry 48:2431–2441. https://doi.org/10.1021/bi802300k
Fan J, Francis F, Liu Y et al (2011) An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet Mol Res 10:3056–3069. https://doi.org/10.4238/2011.December.8.2
Swarup S, Williams TI, Anholt RRH (2011) Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Gene Brain Behav 10:648–657. https://doi.org/10.1111/j.1601-183X.2011.00704.x
Laughlin JD, Ha TS, Jones DNM, Smith DP (2008) Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 133:1255–1265. https://doi.org/10.1016/j.cell.2008.04.046
Gomez-Diaz C, Reina JH, Cambillau C, Benton R (2013) Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol 11:e1001546. https://doi.org/10.1371/journal.pbio.1001546
Bentzur A, Shmueli A, Omesi L et al (2018) Odorant binding protein 69a connects social interaction to modulation of social responsiveness in Drosophila. PLoS Genet 14:e1007328. https://doi.org/10.1371/journal.pgen.1007328
Matsuo T, Sugaya S, Yasukawa J et al (2007) Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol 5:0985–0996. https://doi.org/10.1371/journal.pbio.0050118
Sun JS, Larter NK, Chahda S et al (2018) Humidity response depends on the small soluble protein Obp59a in Drosophila. eLife7 7:39249. https://doi.org/10.7554/eLife.39249
Larter NK, Sun JS, Carlson JR (2016) Organization and function of Drosophila odorant binding proteins. Elife 5:e20242. https://doi.org/10.7554/eLife.20242
Briand L, Perez V, Huet JC et al (1999) Optimization of the production of a honeybee odorant-binding protein by Pichia pastoris. Protein Expr Purif 15:362–369. https://doi.org/10.1006/prep.1998.1027
Briand L, Swasdipan N, Nespoulous C et al (2002) Characterization of a chemosensory protein (ASP3c) from honeybee (Apis mellifera L.) as a brood pheromone carrier. Eur J Biochem 269:4586–4596. https://doi.org/10.1046/j.1432-1033.2002.03156.x
Qiao H, He X, Schymura D et al (2011) Cooperative interactions between odorant-binding proteins of Anopheles gambiae. Cell Mol Life Sci 68:1799–1813. https://doi.org/10.1007/s00018-010-0539-8
Stensmyr MC, Dweck HKM, Farhan A et al (2012) A Conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151:1345–1357. https://doi.org/10.1016/j.cell.2012.09.046
Dweck HKM, Ebrahim SAM, Khallaf MA et al (2016) Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. Elife 5:e14925
Vogt RG, Rybczynski R, Lerner MR (1991) Molecular cloning and sequencing of general odorant-binding proteins GOBP1 and GOBP2 from the tobacco hawk moth Manduca sexta: comparisons with other insect OBPs and their signal peptides. J Neurosci 11:2972–2984
Pelosi P, Maida R (1995) Odorant-binding proteins in insects. Comp Biochem Physiol B Biochem Mol Biol 111:503–514. https://doi.org/10.1016/0305-0491(95)00019-5
Steinbrecht RA, Laue M, Ziegelberger G (1995) Immunolocalization of insect odorant-binding proteins—a comparative-study. Chem Senses 20:109–110
Krieger J, von Nickisch-Rosenegk E, Mameli M et al (1996) Binding proteins from the antennae of Bombyx mori. Insect Biochem Mol Biol 26:297–307. https://doi.org/10.1016/0965-1748(95)00096-8
Sun JS, Xiao S, Carlson JR (2018) The diverse small proteins called odorant-binding proteins. R Soc Open Biol 8:180–208. https://doi.org/10.1098/rsob.180208
Ban L, Scaloni A, D’Ambrosio C et al (2003) Biochemical characterization and bacterial expression of an odorant-binding protein from Locusta migratoria. Cell Mol Life Sci 60:390–400. https://doi.org/10.1007/s000180300032
Honson N, Johnson MA, Oliver JE et al (2003) Structure-activity studies with pheromone-binding proteins of the gypsy moth, Lymantria dispar. Chem Senses 28:479–489. https://doi.org/10.1093/chemse/28.6.479
Andronopoulou E, Labropoulou V, Douris V et al (2006) Specific interactions among odorant-binding proteins of the African malaria vector Anopheles gambiae. Insect Mol Biol 15:797–811. https://doi.org/10.1111/j.1365-2583.2006.00685.x
Lartigue A, Gruez A, Spinelli S et al (2003) The Crystal structure of a cockroach pheromone-binding protein suggests a new ligand binding and release mechanism. J Biol Chem 278:30213–30218. https://doi.org/10.1074/jbc.m304688200
Spinelli S, Lagarde A, Iovinella I et al (2012) Crystal structure of Apis mellifera OBP14, a C-minus odorant-binding protein, and its complexes with odorant molecules. Insect Biochem Mol Biol 42:41–50. https://doi.org/10.1016/j.ibmb.2011.10.005
Tsitsanou KE, Thireou T, Drakou CE et al (2012) Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents. Cell Mol Life Sci 69:283–297. https://doi.org/10.1007/s00018-011-0745-z
Drakou CE, Tsitsanou KE, Potamitis C et al (2016) The crystal structure of the AgamOBP1•Icaridin complex reveals alternative binding modes and stereo-selective repellent recognition. Cell Mol Life Sci 74:319–338. https://doi.org/10.1007/s00018-016-2335-6
Mao Y, Xu X, Xu W et al (2010) Crystal and solution structures of an odorant-binding protein from the southern house mosquito complexed with an oviposition pheromone. Proc Natl Acad Sci 107:19102–19107. https://doi.org/10.1073/pnas.1012274107
Damberger FF, Michel E, Ishida Y et al (2013) Pheromone discrimination by a pH-tuned polymorphism of the Bombyx mori pheromone-binding protein. Proc Natl Acad Sci 110:18680–18685. https://doi.org/10.1073/pnas.1317706110
Leite NR, Krogh R, Xu W et al (2009) Structure of an odorant-binding protein from the mosquito Aedes aegypti suggests a binding pocket covered by a pH-sensitive “Lid”. PLoS One 4:e8006. https://doi.org/10.1371/journal.pone.0008006
Ziemba BP, Murphy EJ, Edlin HT, Jones DNM (2013) A novel mechanism of ligand binding and release in the odorant binding protein 20 from the malaria mosquito Anopheles gambiae. Protein Sci 22:11–21. https://doi.org/10.1002/pro.2179
Shiota Y, Sakurai T, Daimon T et al (2018) In vivo functional characterisation of pheromone binding protein-1 in the silkmoth, Bombyx mori. Sci Rep 8:13529. https://doi.org/10.1038/s41598-018-31978-2
Danty E, Briand L, Michard-Vanhée C et al (1999) Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J Neurosci 19:7468–7475. https://doi.org/10.1523/JNEUROSCI.19-17-07468.1999
Schägger H (2006) Tricine–SDS-PAGE. Nat Protoc 1:16–22. https://doi.org/10.1038/nprot.2006.4
Whitmore L, Wallace BA (2004) DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucl Acids Res 32:W668–W673. https://doi.org/10.1093/nar/gkh371
Kabsch W (2010) XDS. Acta Crystallogr Sect D Biol Crystallogr 66:125–132. https://doi.org/10.1107/S0907444909047337
Keegan RM, Winn MD (2007) MrBUMP: an automated pipeline for molecular replacement. Acta Crystallogr Sect D: Biol Crystallogr 64:119–124. https://doi.org/10.1107/S0907444907037195
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr Sect D: Biol Crystallogr 60:2126–2132. https://doi.org/10.1107/S0907444904019158
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr Sect D: Biol Crystallogr 53:240–255. https://doi.org/10.1107/S0907444996012255
Adams PD, Grosse-Kunstleve RW, Hung LW et al (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr Sect D: Biol Crystallogr 58:1948–1954. https://doi.org/10.1107/S0907444902016657
Urzhumtseva L, Afonine PV, Adams PD, Urzhumtsev A (2009) Crystallographic model quality at a glance. Acta Crystallogr Sect D: Biol Crystallogr 65:297–300. https://doi.org/10.1107/S0907444908044296
van Aalten DMF, Bywater R, Findlay JB et al (1996) PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J Comput Aided Mol Des 10:255–262. https://doi.org/10.1007/BF00355047
Liang J, Edelsbrunner H, Woodward C (1998) Anatomy of protein pockets and cavities: measurement of binding site geometry and implications for ligand design. Protein Sci 7:1884–1897. https://doi.org/10.1002/pro.5560070905
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Stensmyr MC, Dekker T, Hansson BS (2003) Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc R Soc B Biol Sci 270:2333–2340. https://doi.org/10.1098/rspb.2003.2512
Acknowledgements
We thank Dr. J. R. Carlson for Drosophila lines; Dr. C. Everaerts for help with the statistics; and Dr. T. Tanimura for discussion and critical reading. The ESRF is acknowledged for access to beamlines via its in-house research program. Mass spectrometry experiments were performed by the Plateforme d’Analyse Protéomique de Paris Sud-Ouest (PAPPSO, Micalis Institute, INRA, AgroParisTech, Université Paris-Saclay, 78350 Jouy-en-Josas, France).
Funding
This work was partly supported by grants from the Institut National de la Recherche Agronomique, of the Centre National de la Recherche Scientifique, of the Université de Bourgogne-Franche Comté, the Bourgogne-Franche Comté Regional Council (PARI 2010–2011–2012, AGRALE1 Project), and a postdoctoral fellowship from the Bourgogne Regional Council (D.G.). Fellowship for PhD to K.R. (INRA + Bourgogne-Franche Comté Regional Council).
Author information
Authors and Affiliations
Contributions
JFF and LB designed the research. DG, KR, FN, NP, SF, GG, and TC performed the research. DG, KR, FN, SF, GG, TC, and MM analysed the data. The manuscript was written by DG, KR, JFF, and LB. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gonzalez, D., Rihani, K., Neiers, F. et al. The Drosophila odorant-binding protein 28a is involved in the detection of the floral odour ß-ionone. Cell. Mol. Life Sci. 77, 2565–2577 (2020). https://doi.org/10.1007/s00018-019-03300-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03300-4