Abstract
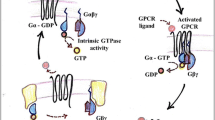
G-protein βγ subunits are key participants in G-protein signaling. These subunits facilitate interactions between receptors and G proteins that are critical for the G protein activation cycle at the plasma membrane. In addition, they play roles in directly transducing signals to an ever expanding range of downstream targets, including integral membrane and cytosolic proteins. Emerging data indicate that Gβγ may play additional roles at intracellular compartments including endosomes, the Golgi apparatus, and the nucleus. Here, we discuss the molecular and structural basis for their ability to coordinate this wide range of cellular activities.




Similar content being viewed by others
References
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Oldham WM, Hamm E (2006) Structural basis of function in heterotrimeric G proteins. Q Rev Biophys 39(02):117–166
Rasmussen SGF, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS et al (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477(7366):549–555
Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH et al (2015) Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348(6241):1361–1365
Oldham WM, Hamm HE (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9(1):60–71
Clapham DE, Neer EJ (1997) G protein βγ subunits. Annu Rev Pharmacol Toxicol 37:167–203
Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA et al (2001) Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors. EMBO J 20(4):767–776
Smrcka AV (2008) G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci 65(14):2191–2214
Neer EJ, Schmidt CJ, Nambudripad R, Smith TF (1994) The ancient regulatory-protein family of WD-repeat proteins. Nature 371(6495):297–300
Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB (1996) The 2.0 Å crystal structure of a heterotrimeric G protein. Nature 379(6563):311–319
Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB (1996) Crystal structure of a G-protein βγ dimer at 2.1Å resolution. Nature 379(6563):369–374
Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG et al (1995) The structure of the G protein heterotrimer Giα1β1γ2. Cell 83(6):1047–1058
Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24(5):181–185
Mumby SM, Casey PJ, Gilman AG, Gutowski S, Sternweis PC (1990) G protein γ subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci USA 87:5873–5877
Akgoz M, Kalyanaraman V, Gautam N (2006) G protein βγ complex translocation from plasma membrane to Golgi complex is influenced by receptor γ subunit interaction. Cell Signal 18(10):1758–1768
Wedegaertner PB, Wilson PT, Bourne HR (1995) Lipid modifications of trimeric G proteins. J Biol Chem 270(2):503–506
Snow BE, Krumins AM, Brothers GM, Lee SF, Wall MA, Chung S et al (1998) A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc Natl Acad Sci USA 95(22):13307–13312
Witherow DS, Wang Q, Levay K, Cabrera JL, Chen J, Willars GB et al (2000) Complexes of the G protein subunit gβ5 with the regulators of G protein signaling RGS7 and RGS9. Characterization in native tissues and in transfected cells. J Biol Chem 275(32):24872–24880
Gautam N, Northup J, Tamir H, Simon MI (1990) G protein diversity is increased by associations with a variety of gamma subunits. Proc Natl Acad Sci USA 87(20):7973–7977
Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC et al (2013) The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65(2):545–577
Neer EJ, Pulsifer L, Wolf LG (1988) The amino terminus of G protein α subunits is required for interaction with βγ. J Biol Chem 263(18):8970–8996
Denker BM, Neer EJ, Schmidt CJ (1992) Mutagenesis of the amino terminus of the α subunit of the G protein Go. In vitro characterization of αo-βγ interactions. J Biol Chem 267(9):6272–6277
Sarvazyan NA, Remmers AE, Neubig RR (1998) Determinants of Giα and βγ binding: measuring high affinity interactions in a lipid environment using flow cytometry. J Biol Chem 273(14):7934–7940
Sprang SR (1997) G protein mechanisms: insights from structural analysis. Ann Rev Biochem 66:639–678
Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M et al (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13(9):778–786
Klein S, Reuveni H, Levitzki A (2000) Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci USA 97(7):3219–3223
Florio VA, Sternweis PC (1989) Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem 264:3909–3915
Carpenter B, Tate CG (2016) Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation. Protein Eng Des Sel 29(12):583–594
Nehme R, Carpenter B, Singhal A, Strege A, Edwards PC, White CF et al (2017) Mini-G proteins: novel tools for studying GPCRs in their active conformation. PLoS One 12(4):e0175642
Wan Q, Okashah N, Inoue A, Nehme R, Carpenter B, Tate CG et al (2018) Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J Biol Chem 293(19):7466–7473
Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J et al (2017) Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546(7656):118–123
Liang YL, Khoshouei M, Glukhova A, Furness SGB, Zhao P, Clydesdale L et al (2018) Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555(7694):121–125
Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q et al (2017) Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546(7657):248–253
Zhao L-H, Ma S, Sutkeviciute I, Shen D-D, Zhou XE, de Waal PW et al (2019) Structure and dynamics of the active human parathyroid hormone receptor-1. Science 364:148–153
Tsai C-J, Marino J, Adaixo RJ, Pamula F, Muehle J, Maeda S, et al. (2019) Cryo-EM structure of the rhodopsin-Gαi-βγ complex reveals binding of the rhodopsin C-terminal tail to the Gβ subunit. Elife. https://doi.org/10.7554/eLife.46041
Wu GY, Bogatkevich GS, Mukhin YV, Benovic JL, Hildebrandt JD, Lanier SM (2000) Identification of Gβγ binding sites in the third intracellular loop of the M-3-muscarinic receptor and their role in receptor regulation. J Biol Chem 275(12):9026–9034
Taylor JM, Jacob-Mosier GG, Lawton RG, VanDort M, Neubig RR (1996) Receptor and membrane interaction sites on Gβ. A receptor- derived peptide binds to the carboxyl terminus. J Biol Chem 271(7):3336–3339
Kan W, Adjobo-Hermans M, Burroughs M, Faibis G, Malik S, Tall GG et al (2014) M3 muscarinic receptor interaction with phospholipase C β3 determines its signaling efficiency. J Biol Chem 289(16):11206–11218
Dupre DJ, Robitaille M, Rebois RV, Hebert TE (2009) The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol 49(1):31–56
Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shektar LR et al (1998) Molecular basis for interactions of G protein βγ subunits with effectors. Science 280:1271–1274
Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300(5623):1256–1262
Whorton MR, MacKinnon R (2013) X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498(7453):190–197
Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J et al (1995) A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits. Science 268(5214):1166–1169
Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE (1998) Gβ binding to GIRK4 subunit is critical for G protein-gated K + channel activation. J Biol Chem 273(27):16946–16952
Sankaran B, Osterhout J, Wu D, Smrcka AV (1998) Identification of a structural element in phospholipase C β2 that interacts with G protein βγ subunits. J Biol Chem 273(12):7148–7154
Davis TL, Bonacci TM, Sprang SR, Smrcka AV (2005) Structural and molecular characterization of a preferred protein interaction surface on G protein βγ subunits. Biochemistry 44(31):10593–10604
Clackson T, Ultsch MH, Wells JA, de Vos AM (1998) Structural and functional analysis of the 1: 1 growth hormone: receptor complex reveals the molecular basis for receptor affinity. J Mol Biol 277(5):1111–1128
Fairbrother WJ, Christinger HW, Cochran AG, Fuh G, Keenan CJ, Quan C et al (1998) Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry 37(51):17754–17764
Ma B, Wolfson HJ, Nussinov R (2001) Protein functional epitopes: hot spots, dynamics and combinatorial libraries. Curr Opin Struct Biol 11(3):364–369
Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC et al (1989) The STE4 and STE18 genes of yeast encode potential b and g subunits of the mating factor receptor-coupled G protein. Cell 56:467–477
Leberer E, Dignard D, Hougan L, Thomas DY, Whiteway M (1992) Dominant-negative mutants of a yeast G-protein β subunit identify two functional regions involved in pheromone signalling. EMBO J 11:4805–4813
Leeuw T, Wu C, Schrag JD, Whiteway M, Thomas DY, Leberer E (1998) Interaction of a G-protein βγ subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature 391(6663):191–195
Bonacci TM, Ghosh M, Malik S, Smrcka AV (2005) Regulatory interactions between the amino terminus of G-protein βγ subunits and the catalytic domain of PLC β2. J Biol Chem 280:10174–10181
Brand CS, Sadana R, Malik S, Smrcka AV, Dessauer CW (2015) Adenylyl cyclase 5 regulation by Gβγ involves isoform-specific use of multiple interaction sites. Mol Pharmacol 88:758–767
Choudhury S, Baradaran-Mashinchi P, Torres MP (2018) Negative feedback phosphorylation of Gγ subunit Ste18 and the Ste5 scaffold synergistically regulates MAPK activation in yeast. Cell Rep 23(5):1504–1515
Yasuda H, Lindorfer MA, Myung C-S, Garrison JC (1998) Phosphorylation of the G protein γ12 subunit regulates effector specificity. J Biol Chem 273(34):21958–21965
Morishita R, Nakayama H, Isobe T, Matsuda T, Hashimoto Y, Okano T et al (1995) Primary structure of a γ subunit of G protein, γ12, and its phosphorylation by protein kinase C. J Biol Chem 270(49):29469–29475
Dewhurst HM, Choudhury S, Torres MP (2015) Structural analysis of PTM hotspots (SAPH-ire)—a quantitative informatics method enabling the discovery of novel regulatory elements in protein families. Mol Cell Proteom 14:2285–2297
Iniguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG (1992) G protein βγ subunits synthesized in Sf9 cells. J Biol Chem 267:23409–23417
Ueda N, Iniguez-Lluhi JA, Lee E, Smrcka AV, Robishaw JD, Gilman AG (1994) G protein βγ subunits. Simplified purification and properties of novel isoforms. J Biol Chem 269(6):4388–4395
Myung C-S, Yasuda H, Liu WW, Harden TK, Garrison JC (1999) Role of isoprenoid lipids on the heterotrimeric G protein γ subunit in determining effector activation. J Biol Chem 274(23):16595–16603
O’Neill PR, Karunarathne WKA, Kalyanaraman V, Silvius JR, Gautam N (2012) G-protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes. Proc Natl Acad Sci 109(51):E3568–E3577
Ajith Karunarathne WK, O’Neill PR, Martinez-Espinosa PL, Kalyanaraman V, Gautam N (2012) All G protein βγ complexes are capable of translocation on receptor activation. Biochem Biophys Res Commun 421(3):605–611
Saini DK, Kalyanaraman V, Chisari M, Gautam N (2007) A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem 282(33):24099–24108
Senarath K, Payton JL, Kankanamge D, Siripurapu P, Tennakoon M, Karunarathne A (2018) Gγ identity dictates efficacy of Gβγ signaling and macrophage migration. J Biol Chem 293(8):2974–2989
Khan SM, Sung JY, Hébert TE (2016) Gβγ subunits—different spaces, different faces. Pharmacol Res 111:434–441
Garcia-Olivares J, Torres-Salazar D, Owens WA, Baust T, Siderovski DP, Amara SG et al (2013) Inhibition of dopamine transporter activity by G protein βγ subunits. PLoS One 8(3):e59788
Garcia-Olivares J, Baust T, Harris S, Hamilton P, Galli A, Amara SG et al (2017) Gβγ subunit activation promotes dopamine efflux through the dopamine transporter. Mol Psychiatry 22(12):1673–1679
Mauna JC, Harris SS, Pino JA, Edwards CM, DeChellis-Marks MR, Bassi CD et al (2019) G protein βγ subunits play a critical role in the actions of amphetamine. Transl Psychiatry 9(1):81
Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L et al (2012) A Gβγ effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell 22(1):92–103
Wang Y, Xu X, Pan M, Jin T (2016) ELMO1 directly interacts with Gβγ subunit to transduce GPCR signaling to Rac1 activation in chemotaxis. J Cancer 7(8):973–983
Badheka D, Yudin Y, Borbiro I, Hartle CM, Yazici A, Mirshahi T et al (2017) Inhibition of transient receptor potential melastatin 3 ion channels by G-protein βγ subunits. Elife 6:e26147
Quallo T, Alkhatib O, Gentry C, Andersson DA, Bevan S (2017) G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. Elife 6:e26138
Dembla S, Behrendt M, Mohr F, Goecke C, Sondermann J, Schneider FM et al (2017) Anti-nociceptive action of peripheral mu-opioid receptors by G-βγ protein-mediated inhibition of TRPM3 channels. Elife 6:e26280
Ahmed SM, Daulat AM, Meunier A, Angers S (2010) G protein βγ subunits regulate cell adhesion through Rap1a and its effector Radil. J Biol Chem 285(9):6538–6551
Liu L, Aerbajinai W, Ahmed SM, Rodgers GP, Angers S, Parent CA (2012) Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol Biol Cell 23(24):4751–4765
Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT (1994) A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell 77(1):83–93
Stephens LR, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AV et al (1997) The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89(1):105–114
Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D (2000) Roles of PLCβ2 and β3 and PI3K in chemoattractant-mediated signal transduction. Science 287(5455):1046–1049
Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L et al (2000) Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287(5455):1049–1053
Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y et al (1997) Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J Biol Chem 272(39):24252–24256
Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD et al (2012) G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. Sci Signal 5(253):ra89
Maier U, Babich A, Nürnberg B (1999) Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem 274(41):29311–29317
Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF et al (2013) Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs). Proc Natl Acad Sci USA 110(47):18862–18867
Bresnick AR, Backer JM (2019) PI3Kb-A versatile transducer for GPCR, RTK, and small GTPase signaling. Endocrinology 160(3):536–555
Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H et al (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108(6):809–821
Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL et al (2011) P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun 2:555
Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ et al (1999) Gbg-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98(1):59–68
Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V et al (2010) Regulation of Golgi structure and secretion by receptor-induced G protein bg complex translocation. Proc Natl Acad Sci USA 107(25):11417–11422
Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K et al (2003) Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol 160:89–99
Sui JL, Petit-Jacques J, Logothetis DE (1998) Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA 95(3):1307–1312
Romoser V, Ball R, Smrcka AV (1996) Phospholipase C β2 association with phospholipid interfaces assessed by fluorescence resonance energy transfer. G protein βγ subunit-mediated translocation is not required for enzyme activation. J Biol Chem 271(41):25071–25078
Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J (2008) General and versatile autoinhibition of PLC isozymes. Mol Cell 31(3):383–394
Han DS, Golebiewska U, Stolzenberg S, Scarlata SF, Weinstein H (2011) A dynamic model of membrane-bound phospholipase Cβ2 activation by Gβγ subunits. Mol Pharmacol 80(3):434–445
Wang T, Dowal L, El-Maghrabi MR, Rebecchi M, Scarlata S (2000) The pleckstrin homology domain of phospholipase C-beta(2) links the binding of gbetagamma to activation of the catalytic core. J Biol Chem 275(11):7466–7469
Acknowledgements
National Institutes of Health R35GM127303.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smrcka, A.V., Fisher, I. G-protein βγ subunits as multi-functional scaffolds and transducers in G-protein-coupled receptor signaling. Cell. Mol. Life Sci. 76, 4447–4459 (2019). https://doi.org/10.1007/s00018-019-03275-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03275-2