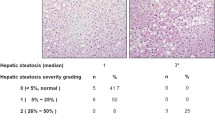
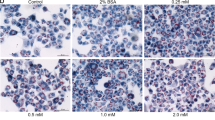
Abstract
High-carbohydrate diets (HCD) can induce the occurrence of nonalcoholic fatty liver disease (NAFLD), characterized by dramatic accumulation of hepatic lipid droplets (LDs). However, the potential molecular mechanisms are still largely unknown. In this study, we investigated the role of autophagy in the process of HCD-induced changes of hepatic lipid metabolism, and to examine the process of underlying mechanisms during these molecular contexts. We found that HCD significantly increased hepatic lipid accumulation and activated autophagy. Using primary hepatocytes, we found that HG increased lipid accumulation and stimulated the release of NEFA by autophagy-mediated lipophagy, and that lipophagy significantly alleviated high glucose (HG)-induced lipid accumulation. Oxidative and endoplasmic reticulum (ER) stress pathways played crucial regulatory roles in HG-induced lipophagy activation and HG-induced changes of lipid metabolism. Further investigation found that HG-activated lipophagy and HG-induced changes of lipid metabolism were via enhancing carbohydrate response element-binding protein (ChREBP) DNA binding capacity at PPARγ promoter region, which in turn induced transcriptional activation of the key genes related to lipogenesis and autophagy. The present study, for the first time, revealed the novel mechanism for lipophagy mediating HCD-induced changes of lipid metabolism by oxidative stress and ER stress, and ChREBP/PPARγ pathways. Our study provided innovative evidence for the direct relationship between carbohydrate and lipid metabolism via ChREBP/PPARγ pathway.







Similar content being viewed by others
Abbreviations
- 3-MA:
-
3-Methyl adenine
- 4-PBA:
-
4-Phenylbutyric acid
- 6PGD:
-
6-Phosphogluconate dehydrogenase
- ACCa:
-
Acetyl-CoA carboxylase a
- ACSL:
-
Acyl-CoA synthetase long-chain
- AO:
-
Acridine orange
- ATF:
-
Activating transcription factor
- ATG:
-
Autophagy-related gene
- BSA:
-
Bovine serum albumin
- CF:
-
Condition factor
- ChREBP:
-
Carbohydrate response element-binding protein
- CPT-1:
-
Carnitine palmitoyltransferase-1
- CQ:
-
Chloroquine
- DCFH2-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- eIF2α:
-
Eukaryotic translation initiation factor 2α
- ERS:
-
Endoplasmic reticulum stress
- FABPL:
-
Fatty acid-binding protein liver
- FAS:
-
Fatty acid synthase
- FBW:
-
Final mean body weight
- FCR:
-
Feed conversion rate
- FFA:
-
Free fatty acids
- FI:
-
Feed intake
- G6PD:
-
Glucose 6-phosphate dehydrogenase
- GLUT:
-
Glucose transporter
- GRP78:
-
Glucose-regulated protein 78
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- HCD:
-
High-carbohydrate diet
- H&E:
-
Hematoxylin and eosin
- HG:
-
High glucose
- HSI:
-
Hepatosomatic index
- HSL:
-
Hormone-sensitive lipase
- ICD:
-
Intermediate carbohydrate diet
- ICDH:
-
Isocitrate dehydrogenase
- IRE1α:
-
Inositol requiring 1α
- LCD:
-
Low-carbohydrate diet
- LD:
-
Lipid droplet
- LXR a:
-
Liver x receptor a
- MAP1LC3B:
-
Microtubule-associated proteins 1A/1B light chain 3B
- IBW:
-
Initial mean body weight
- M199:
-
Medium-199
- MDA:
-
Malondialdehyde
- MDC:
-
Monodansylcadaverine
- ME:
-
Malic enzyme
- NAC:
-
N-acetyl-l-cysteine
- NAFLD:
-
Nonalcoholic fatty liver disease
- NEFA:
-
Nonesterified fatty acid
- OD:
-
Optical density
- ORO:
-
Oil red O
- PERK:
-
Protein kinase R (PKR)-like ER kinase
- PPAR:
-
Peroxisome proliferator-activated receptor
- PVDF:
-
Polyvinylidene difluoride
- ROS:
-
Reactive oxygen species
- S.E.M:
-
Standard error of the mean
- SGR:
-
Specific growth rate
- SOD:
-
Superoxide dismutase
- SREBP-1:
-
Sterol regulatory element-binding proteins-1
- TG:
-
Triglyceride
- UPR:
-
Unfolded protein response
- VAI:
-
Visceral adipose index
- VSI:
-
Viscerosomatic index
- WG:
-
Weight gain
- XBP1:
-
X-box binding protein 1
References
Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51:679–689
Farrell GC, Larter CZ (2006) Non-alcoholic fatty liver: from steatosis to cirrhosis. Hepatology 43:S99–S112
Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51:1961–1971
Neuschwander-Tetri BA (2013) Carbohydrate intake and nonalcoholic fatty liver disease. Curr Opin Clin Nutr 16:446–452
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477
Singh R, Kaushik S, Wang YJ, Xiang YQ, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (2009) Autophagy regulates lipid metabolism. Nature 458:1131–1135
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A (2012) The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56:952–964
Dong HQ, Czaja MJ (2011) Regulation of lipid droplets by autophagy. Trends Endocrinol Metab 22:234–240
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:282041
Lavallard VJ, Gual P (2014) Autophagy and non-alcoholic fatty liver disease. Biomed Res Int 2014:120179
Azad MB, Chen YQ, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11:777–790
Kawai D, Takaki A, Nakatsuka A, Wada J, Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y et al (2012) Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology 56:912–921
Panieri E, Santoro MM (2016) ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7:e2253
Pan YX, Luo Z, Zhuo MQ, Wei CC, Chen GH, Song YF (2018) Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol 199:12–20
Wang H, Sun RQ, Zeng XY, Zhou X, Li SP, Jo E, Molero JC, Ye JM (2015) Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology 156:169–181
Madaro L, Marrocco V, Carnio S, Sandri M, Bouché M (2013) Intracellular signaling in ER stress-induced autophagy in skeletal muscle cells. FASEB J 27:1990–2000
Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J et al (2008) UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15:829–840
Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR et al (2001) Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest 107:1263–1273
Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH (2001) Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr Biol 11:764–768
Zheng JL, Zhuo MQ, Luo Z, Pan YX, Song YF, Huang C, Zhu QL, Hu W, Chen QL (2015) Peroxisome proliferator-activated receptor gamma (PPARγ) in yellow catfish Pelteobagrus fulvidraco: molecular characterization, mRNA expression and transcriptional regulation by insulin in vivo and in vitro. Gen Comp Endocr 212:51–62
Meyer A, Van de Peer Y (2005) From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). BioEssays 27:937–945
Gong G, Dan C, Xiao S, Guo W, Huang P, Xiong Y, Wu J, He Y, Zhang J, Li X et al (2018) Chromosomal-level assembly of yellow catfish genome using third-generation DNA sequencing and Hi-C analysis. GigaScience 7:giy120. https://doi.org/10.1093/gigascience/giy120
Ye WJ, Tan XY, Chen YD, Luo Z (2009) Effects of dietary protein to carbohydrate ratios on growth and body composition of juvenile yellow catfish, Pelteobagrus fulvidraco (Siluriformes, Bagridae, Pelteobagrus). Aquac Res 40:1410–1418
Wei CC, Luo Z, Hogstrand C, Xu YH, Wu LX, Chen GH, Pan YX, Song YF (2018) Zinc reduces hepatic lipid deposition and activates lipophagy via Zn2+/MTF-1/PPARα and Ca2+/CaMKKβ/AMPK pathways. FASEB J 32:6666–6680
Yang SB, Tan XY, Zhang DG, Cheng J, Luo Z (2018) Identification of ten SUMOylation-related genes from yellow catfish Pelteobagrus fulvidraco, and their transcriptional responses to carbohydrate addition in vivo and in vitro. Front Physiol 9:1544
Wu K, Luo Z, Hogstrand C, Chen GH, Wei CC, Li DD (2018) Zn stimulates the phospholipids biosynthesis via the pathways of oxidative and endoplasmic reticulum stress in the intestine of freshwater teleost yellow catfish. Environ Sci Technol 52:9206–9214
Giustarini D, Dalle-donne I, Milzani A, Fanti P, Rossi R (2013) Analysis of GSH and GSSG after derivatization with n-ethylmaleimide. Nat Protoc 8:1660–1669
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena AA, Adachi H, Adams CM, Adams PD, Adeli K et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222
Song YF, Luo Z, Zhang LH, Hogstrand C, Pan YX (2016) Endoplasmic reticulum stress and disturbed calcium homeostasis are involved in copper-induced alteration in hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Chemosphere 144:2443–2453
Cai XY, Liu YL, Hu YQ, Liu XZ, Jiang HY, Yang SH, Shao Z, Xia Y, Xiong L (2018) Ros-mediated lysosomal membrane permeabilization is involved in bupivacaine-induced death of rabbit intervertebral disc cells. Redox Biol 18:65–76
Wu K, Tan XY, Xu YH, Chen GH, Zhuo MQ (2018) Functional analysis of promoters of genes in lipid metabolism and their transcriptional response to STAT3 under leptin signals. Genes 9:334
Xu YH, Luo Z, Wu K, Fan YF, You WJ, Zhang LH (2017) Structure and functional analysis of promoters from two liver isoforms of CPT I in grass carp Ctenopharyngodon idella. Int J Mol Sci 18:E2405
Thorens B (1996) Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol 270:G541–G553
Assumpção JAF, Magalhães KG, Corrêa JR (2017) The role of pparγ and autophagy in ros production, lipid droplets biogenesis and its involvement with colorectal cancer cells modulation. Cancer Cell Int 17:82
Postic C, Dentin R, Denechaud PD, Girard J (2007) ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr 27:179–192
Chen B, Zheng YM, Zhang JP (2018) Comparative study of different diets-induced NAFLD models of zebrafish. Front Endocrinol 9:366
Dias J, Alvarez MJ, Diez A, Arzel J, Corraze G, Bautista JM, Kaushik SJ (1998) Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European sea bass (Dicentrarchus labrax). Aquaculture 161:169–186
Gou R, Chen JT, Sheng SF, Wang RQ, Fang YD, Yang ZJ, Wang L, Tang L (2016) Kim-1 mediates high glucose-induced autophagy and apoptosis in renal tubular epithelial cells. Cell Physiol Biochem 38:2479–2488
Wang H, Sun RQ, Camera D, Zeng XY, Jo E, Chan SM, Herbert TP, Molero JC, Ye JM (2016) Endoplasmic reticulum stress up-regulates Nedd4-2 to induce autophagy. FASEB J 30:2549–2556
Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B (2018) Endoplasmic reticulum stress signaling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 69:927–947
Chen Y, Azad MB, Gibson SB (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16:1040–1052
Zhang Z, Guo M, Zhao S, Shao J, Zheng S (2016) ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic Biol Med 101:272–283
Zhao XJ, Yu HW, Yang YZ, Wu WY, Chen TY, Jia KK, Kang LL, Jiao RQ, Kong LD (2018) Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol 18:124–137
Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, De AF, Sisci D, Mauro L et al (2013) Omega-3 PUFA ethanolamides DHEA and EPA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J Cell Physiol 228:1314–1322
Zhou J, Zhang W, Liang B, Casimiro MC, Whitaker-Menezes D, Wang M, Lisanti MP, Lanza-Jacoby S, Pestell RG, Wang C (2009) PPARγ activation induces autophagy in breast cancer cells. Int J Biochem Cell Biol 41:2334–2342
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFD0900400) and Fundamental Research Funds for the Central Universities, China (Grant no. 2662018PY089).
Author information
Authors and Affiliations
Contributions
ZL and TZ designed the experiments. TZ carried out animal and cell experiments and sample analysis with the help of KW, YHX, GHC, and CCW; TZ, ZL and CH analyzed data; TZ wrote the manuscript, and ZL and CH revised the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, T., Wu, K., Hogstrand, C. et al. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell. Mol. Life Sci. 77, 1987–2003 (2020). https://doi.org/10.1007/s00018-019-03263-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03263-6