Abstract
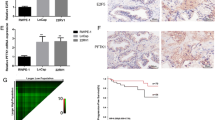
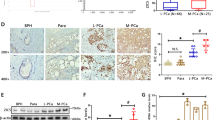
ZNFX1 anti-sense RNA 1 (ZFAS1) has been indicated in the tumorigenesis of various human cancers. However, the role of ZFAS1 in prostate cancer (PCa) progression and the underlying mechanisms remain incompletely understood. In the present study, we discovered that ZFAS1 is upregulated in PCa and that ZFAS1 overexpression predicted poor clinical outcomes. ZFAS1 overexpression notably promoted the proliferation, invasion, and epithelial–mesenchymal transition of PCa cells. Furthermore, we not only discovered that miR-27a/15a/16 are targeted by ZFAS1, which binds to their miRNA-response elements, but also revealed their tumor suppressor roles in PCa. We also identified that the Hippo pathway transducer YAP1, as well as its cooperator, TEAD1, are common downstream targets of miR-27a/15a/16. In addition, H3K9 demethylase KDM3A was found to be another target gene of miR-27a. Importantly, YAP1, TEAD1, and KDM3A all act as strong c-Myc inducers in an androgen-independent manner. Taken together, we suggest a regulatory network in which ZFAS1 is capable of enhancing c-Myc expression by inducing the expression of YAP1, TEAD1, and KDM3A through crosstalk with their upstream miRNAs, thereby globally promoting prostate cancer tumorigenesis.








Similar content being viewed by others
Abbreviations
- ZFAS1:
-
ZNFX1 anti-sense RNA 1
- YAP1:
-
Yes-associated protein 1
- TEAD1:
-
TEA domain transcription factor 1
- KDM3A:
-
Lysine demethylase 3A
- PCa:
-
Prostate cancer
- ceRNA:
-
Competing endogenous RNA
- AR:
-
Androgen receptor
- PSA:
-
Prostate-specific antigen
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Barry MJ, Simmons LH (2017) Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am 101(4):787–806
Robinson D, Van Allen EM, Wu YM et al (2015) Integrative clinical genomics of advanced prostate cancer. Cell 162(2):454
Mitsiades N (2013) A road map to comprehensive androgen receptor axis targeting for castration-resistant prostate cancer. Cancer Res 73(15):4599–4605
Sharma NL, Massie CE, Ramos-Montoya A et al (2013) The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23(1):35–47
Tsai MC, Spitale RC, Chang HY (2011) Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res 71(1):3–7
Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154(1):26–46
Wang K, Liu CY, Zhou LY et al (2015) APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun 6:6779
Xue X, Yang YA, Zhang A et al (2016) LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 35(21):2746–2755
Sun M, Nie F, Wang Y et al (2016) LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1. Cancer Res 76(21):6299–6310
Xue M, Chen W, Xiang A et al (2017) Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer 16(1):143
Wang H, Huo X, Yang XR et al (2017) STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol Cancer 16(1):136
Du Z, Sun T, Hacisuleyman E et al (2016) Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nat Commun 7:10982
Karreth FA, Reschke M, Ruocco A et al (2015) The BRAF pseudogene functions as a competitive endogenous RNA and induces lymphoma in vivo. Cell 161(2):319–332
Tay Y, Kats L, Salmena L et al (2011) Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147(2):344–357
Song YX, Sun JX, Zhao JH et al (2017) Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun 8(1):289
Ding J, Yeh CR, Sun Y et al (2018) Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene 37(37):5037–5053
Chakravarty D, Sboner A, Nair SS et al (2014) The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5:5383
Zhang A, Zhao JC, Kim J et al (2015) LncRNA HOTAIR enhances the androgen-receptor-mediated transcriptional program and drives castration-resistant prostate cancer. Cell Rep 13(1):209–221
Askarian-Amiri ME, Crawford J, French JD et al (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 17(5):878–891
Zhou H, Wang F, Chen H et al (2016) Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging (Albany NY) 8(9):2023–2038
Li T, Xie J, Shen C et al (2015) Amplification of long noncoding RNA ZFAS1 promotes metastasis in hepatocellular carcinoma. Cancer Res 75(15):3181–3191
Wang W, Xing C (2016) Upregulation of long noncoding RNA ZFAS1 predicts poor prognosis and prompts invasion and metastasis in colorectal cancer. Pathol Res Pract 212(8):690–695
Liu R, Zeng Y, Zhou CF et al (2017) Long noncoding RNA expression signature to predict platinum-based chemotherapeutic sensitivity of ovarian cancer patients. Sci Rep 7(1):18
Dang CV (2012) MYC on the path to cancer. Cell 149(1):22–35
Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV (2015) MYC and metabolism on the path to cancer. Semin Cell Dev Biol 43:11–21
Gil J, Kerai P, Lleonart M et al (2005) Immortalization of primary human prostate epithelial cells by c-Myc. Cancer Res 65(6):2179–2185
Ellwood-Yen K, Graeber TG, Wongvipat J et al (2003) Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4(3):223–238
Hubbard GK, Mutton LN, Khalili M et al (2016) Combined MYC activation and Pten loss are sufficient to create genomic instability and lethal metastatic prostate cancer. Cancer Res 76(2):283–292
Xiang JF, Yin QF, Chen T et al (2014) Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24(5):513–531
Xiao ZD, Han L, Lee H et al (2017) Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun 8(1):783
Fan L, Peng G, Sahgal N et al (2016) Regulation of c-Myc expression by the histone demethylase JMJD1A is essential for prostate cancer cell growth and survival. Oncogene 35(19):2441–2452
Chandrashekar DS, Bashel B, Sah B et al (2017) UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19(8):649–658
Paraskevopoulou MD, Vlachos IS, Karagkouni D et al (2016) DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res 44(D1):D231–D238
Vlachos IS, Zagganas K, Paraskevopoulou MD et al (2015) DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43(1):460–466
Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19(4):491–505
Zanconato F, Cordenonsi M, Piccolo S (2016) YAP/TAZ at the roots of cancer. Cancer Cell 29(6):783–803
Moroishi T, Hansen CG, Guan KL (2015) The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15(2):73–79
Bonci D, Coppola V, Musumeci M et al (2008) The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14(11):1271–1277
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20
Liu-Chittenden Y, Huang B, Shim JS et al (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26(12):1300–1305
Koontz LM, Liu-Chittenden Y, Yin F et al (2013) The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell 25(4):388–401
Santucci M, Vignudelli T, Ferrari S et al (2015) The hippo pathway and YAP/TAZ-TEAD protein–protein interaction as targets for regenerative medicine and cancer treatment. J Med Chem 58(12):4857–4873
Jiao S, Wang H, Shi Z et al (2014) A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25(2):166–180
Wang Z, Wu Y, Wang H et al (2014) Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA 111(1):E89–E98
Wang L, Shi S, Guo Z et al (2013) Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One 8(6):e65539
Goda S, Isagawa T, Chikaoka Y, Kawamura T, Aburatani H (2013) Control of histone H3 lysine 9 (H3K9) methylation state via cooperative two-step demethylation by Jumonji domain containing 1A (JMJD1A) homodimer. J Biol Chem 288(52):36948–36956
Gurel B, Iwata T, Koh CM et al (2008) Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol 21(9):1156–1167
Koh CM, Bieberich CJ, Dang CV, Nelson WG, Yegnasubramanian S, De Marzo AM (2010) MYC and prostate cancer. Genes Cancer 1(6):617–628
Cho H, Herzka T, Zheng W et al (2014) RapidCaP, a novel GEM model for metastatic prostate cancer analysis and therapy, reveals myc as a driver of Pten-mutant metastasis. Cancer Discov 4(3):318–333
Wang J, Kobayashi T, Floc’h N et al (2012) B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer. Cancer Res 72(18):4765–4776
Kim J, Roh M, Doubinskaia I, Algarroba GN, Eltoum IE, Abdulkadir SA (2012) A mouse model of heterogeneous, c-MYC-initiated prostate cancer with loss of Pten and p53. Oncogene 31(3):322–332
Zhang P, Cao L, Fan P, Mei Y, Wu M (2016) LncRNA-MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting Fbxw7-mediated c-Myc degradation. EMBO Rep 17(8):1204–1220
Kawasaki Y, Komiya M, Matsumura K et al (2016) MYU, a target lncRNA for Wnt/c-Myc signaling, mediates induction of CDK6 to promote cell cycle progression. Cell Rep 16(10):2554–2564
Chen X, Yang C, Xie S, Cheung E (2018) Long non-coding RNA GAS5 and ZFAS1 are prognostic markers involved in translation targeted by miR-940 in prostate cancer. Oncotarget 9:1048–1062
Aqeilan RI, Calin GA, Croce CM (2010) miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 17(2):215–220
Bonci D, Coppola V, Patrizii M et al (2016) A microRNA code for prostate cancer metastasis. Oncogene 35(9):1180–1192
Chang TC, Yu D, Lee YS et al (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40(1):43–50
Xue G, Yan HL, Zhang Y et al (2015) c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2α and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene 34(11):1393–1406
Tang W, Yu F, Yao H et al (2014) miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene 33(20):2629–2638
Colangelo T, Polcaro G, Ziccardi P et al (2016) The miR-27a-calreticulin axis affects drug-induced immunogenic cell death in human colorectal cancer cells. Cell Death Dis 7:e2108
Sun Y, Yang X, Liu M, Tang H (2016) B4GALT3 up-regulation by miR-27a contributes to the oncogenic activity in human cervical cancer cells. Cancer Lett 375(2):284–292
Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL (2012) Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet 21(14):3112–3127
Mo W, Zhang J, Li X et al (2013) Identification of novel AR-targeted microRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer. PLoS One 8(2):e56592
Wan X, Huang W, Yang S et al (2016) Androgen-induced miR-27A acted as a tumor suppressor by targeting MAP2K4 and mediated prostate cancer progression. Int J Biochem Cell Biol 79:249–260
Acknowledgements
This work was financially supported by grant from the National Natural Science Foundation of China (Grant No. 81702505). The funding bodies played no role in the design or interpretation of the study.
Author information
Authors and Affiliations
Contributions
XC and CP conceived and designed the study. XC and CP performed the experiments. XC and CL analyzed the data and prepared the manuscript. ZZ and X Liu contributed the experimental reagents and materials. X Lin did the pathological diagnosis and provided the pathological sections. All authors have read and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, X., Piao, C., Lv, C. et al. ZNFX1 anti-sense RNA 1 promotes the tumorigenesis of prostate cancer by regulating c-Myc expression via a regulatory network of competing endogenous RNAs. Cell. Mol. Life Sci. 77, 1135–1152 (2020). https://doi.org/10.1007/s00018-019-03226-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03226-x