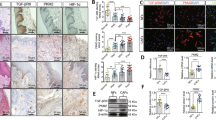
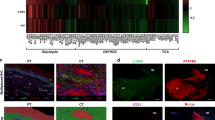
Abstract
Cancers show a metabolic shift towards aerobic glycolysis. By “corrupting” their microenvironment, carcinoma cells are able to obtain energy substrates to “fuel” their mitochondrial metabolism and cell growth in an autophagy-associated, paracrine manner. However, the metabolic changes and role of normal fibroblasts in this process remain unclear. We devised a novel, indirect co-culture system to elucidate the mechanisms of metabolic coupling between stromal cells and oral squamous cell carcinoma (OSCC) cells. Here, we showed that normal oral fibroblasts (NOFs) and OSCC become metabolically coupled through several processes before acquiring an activated phenotype and without inducing senescence. We observed, for the first time, that NOFs export mitochondria towards OSCCs through both direct contact and via indirect mechanisms. NOFs are activated and are able to acquire a cancer-associated fibroblasts metabolic phenotype when co-cultivation with OSSC cells, by undergoing aerobic glycolysis, secreting more reactive oxygen species (ROS), high l-lactate and overexpressing lactate exporter MCT-4, leading to mitochondrial permeability transition pore (mPTP) opening, hypoxia, and mitophagy. On the other hand, Cav-1-low NOFs generate l-lactate to “fuel” mitochondrial metabolism and anabolic growth of OSCC. Most interestingly, the decrease in AMPK activity and PGC-1α expression might involve in regulation of ROS that functions to maintain final energy and metabolic homeostasis. This indicated, for the first time, the existence of ATP and ROS homeostasis during carcinogenesis. Our study suggests that an efficient therapeutical approach has to target the multiple mechanisms used by them to corrupt the normal surrounding stroma and metabolic homeostasis.








Similar content being viewed by others
Availability of data and material
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- NOFs:
-
Normal oral fibroblasts
- CAFs:
-
Cancer-associated fibroblasts
- ROS:
-
Reactive oxygen species
- mPTP:
-
Mitochondrial permeability transition pore
- NOKs:
-
Normal oral epithelial cells
- FBS:
-
Fetal bovine serum
- BPE:
-
Bovine pituitary extract
- KSFM:
-
Serum-free keratinocyte specific medium
- DOK:
-
Dysplastic oral keratinocyte
- EGF:
-
Epidermal growth factor
- MTG:
-
MitoTracker Green
- TMRE:
-
Tetramethylrhodamine, ethyl ester
- FCCP:
-
Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- PBS:
-
Phosphate-buffered saline
- MTDR:
-
MitoTracker Deep Red
- ROS:
-
Reactive oxygen stress
- DCFDA:
-
2′,7′-Dichlorofluorescin diacetate
- ATP:
-
Adenosine triphosphate
- ATPases:
-
ATP-degrading enzymes
- IHC:
-
Immunohistochemistry staining
- EMU:
-
Epithelium–stroma unit
- TNTs:
-
Tunneling nanotubes
- mPTP:
-
Mitochondrial permeability transition pore
- VDAC:
-
Voltage-dependent anion channel
- ECM:
-
Extracellular matrix
- MCTs:
-
Monocarboxylate transporters
- Alpha-SMA:
-
Alpha-smooth muscle actin
- FAP:
-
Fibroblast activation protein
References
Johnson NW, Jayasekara P, Amarasinghe AA (2011) Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 57:19–37
Schafer M, Werner S (2008) Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9:628–638
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8:3984–4001
Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ (2004) Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 90:822–832
Davalos AR, Coppe JP, Campisi J, Desprez PY (2010) Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29:273–283
Rosenthal E, McCrory A, Talbert M, Young G, Murphy-Ullrich J, Gladson C (2004) Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog 40:116–121
Costea DE, Hills A, Osman AH, Thurlow J, Kalna G, Huang X, Pena Murillo C, Parajuli H, Suliman S, Kulasekara KK, Johannessen AC, Partridge M (2013) Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res 73:3888–3901
Jensen DH, Therkildsen MH, Dabelsteen E (2015) A reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblasts. J Oral Pathol Med 44:714–721
Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, McMahon KA, Parton RG, Hill MM, Del Pozo MA, Kim YS, Shen HM (2015) Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy. 11:769–784
Vered M, Lehtonen M, Hotakainen L, Pirila E, Teppo S, Nyberg P, Sormunen R, Zlotogorski-Hurvitz A, Salo T, Dayan D (2015) Caveolin-1 accumulation in the tongue cancer tumor microenvironment is significantly associated with poor prognosis: an in vivo and in vitro study. BMC Cancer 15:25
Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Pavlides S, Gandara R, Pestell RG, Sotgia F, Philp NJ, Lisanti MP (2011) Evidence for a stromal-epithelial “lactate shuttle” in human tumors: mCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 10:1772–1783
Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A (2013) Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med 11:94
Chatterjee M, Ben-Josef E, Thomas DG, Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA, Chen CS, Ludwig T, Bekaii-Saab T, Chakravarti A, Williams TM (2015) Caveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancer. Sci Rep 5:10867
Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, Allen KG, Danilo C, Sotgia F, Bonuccelli G, Jasmin J-F, Xu H, Bosco E, Aronow B, Witkiewicz AK, Pestell RG, Knudsen ES, Lisanti MP (2014) Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 down-regulation and RB tumor suppressor functional inactivation: implications for the response to hormonal therapy. Cancer Biol Ther 7:1212–1225
Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP (2012) Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal 16:1264–1284
Wilde L, Roche M, Domingo-Vidal M, Tanson K, Philp N, Curry J, Martinez-Outschoorn U (2017) Metabolic coupling and the Reverse Warburg Effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol 44:198–203
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950
Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J, Jones RG (2017) AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell reports. 21:1–9
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Rabinovitch RC et al (2017) AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep 21(1):1–9
Yang G et al (2006) The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA 103(44):16472–16477
Capparelli C et al (2012) CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle 11(19):3599–3610
Quijano C et al (2012) Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 11(7):1383–1392
James EL et al (2015) Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res 14(4):1854–1871
Audet-Walsh E, Papadopoli DJ, Gravel SP, Yee T, Bridon G, Caron M, Bourque G, Giguere V, St-Pierre J (2016) The PGC-1alpha/ERRalpha axis represses one-carbon metabolism and promotes sensitivity to anti-folate therapy in breast cancer. Cell Rep 14:920–931
Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98:12072–12077
Para R et al (2019) Metabolic reprogramming as a driver of fibroblast activation in pulmonary fibrosis. Am J Med Sci 357(5):394–398
Hassona Y, Cirillo N, Lim KP, Herman A, Mellone M, Thomas GJ, Pitiyage GN, Parkinson EK, Prime SS (2013) Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-beta. Carcinogenesis 34:1286–1295
Berridge MV, Crasso C, Neuzil J (2015) Mitochondrial genome transfer to tumor cells breaks the rules and establishes a new precedent in cancer biology. Mol Cell Oncol 5:e1023929
Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS (2012) mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11:401–414
Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S (2000) The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 350(Pt 1):219–227
Shan T, Lu H, Ji H, Li Y, Guo J, Chen X, Wu T (2014) Loss of stromal caveolin-1 expression: a novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancer. PLoS One 9:e97239
Folgueira MA, Maistro S, Katayama ML, Roela RA, Mundim FG, Nanogaki S, de Bock GH, Brentani MM (2013) Markers of breast cancer stromal fibroblasts in the primary tumour site associated with lymph node metastasis: a systematic review including our case series. Biosci Rep 33:e00085
Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, Jeong SI, Kang MJ, Kim NH, Kim HJ, Chi SG (2012) Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res 72:4097–4109
Zhao X, He Y, Gao J, Fan L, Li Z, Yang G, Chen H (2013) Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One 8:e59102
Acknowledgements
We thank Hanne Linda Nakkestad for excellent technical assistance. We also thank Molecular Imaging Centre (MIC) for outstanding facilities.
Funding
This work was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme (DEC, project number 22325), the Western Health Authority (DEC grant nr. 911902/2013).
Author information
Authors and Affiliations
Contributions
XL, DEC, ZYZ, and JZG: conceptualization; XL, DEC, ZYZ, and JZG: methodology; DS, ZYZ, ZJG, LAB, RD, and SR: investigation; ZYZ and ZJG: writing—original draft; XL, DEC, ZYZ, LAB, and LJL: writing—review and editing; DEC and LAB: funding acquisition; HD, HP, SS, and LJL: resources; XL and DEC: supervision.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethics approval
The project was approved by the Committee for Ethics in Health Research of West Norway (REK nr. 2010/481); the study was performed in accordance with the Declaration of Helsinki. Tissues were acquired with written informed consent from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Gao, Z., Rajthala, S. et al. Metabolic reprogramming of normal oral fibroblasts correlated with increased glycolytic metabolism of oral squamous cell carcinoma and precedes their activation into carcinoma associated fibroblasts. Cell. Mol. Life Sci. 77, 1115–1133 (2020). https://doi.org/10.1007/s00018-019-03209-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03209-y