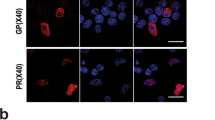
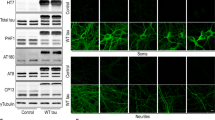
Abstract
The Tar DNA-Binding Protein 43 (TDP-43) and its phosphorylated isoform (pTDP-43) are the major components associated with ubiquitin positive/Tau-negative inclusions found in neurons and glial cells of patients suffering of amyotrophic lateral sclerosis (ALS) or frontotemporal lobar degeneration-TDP-43 (FTLD–TDP). Many studies have revealed that TDP-43 is also in the protein inclusions associated with neurodegenerative conditions other than ALS and FTLD–TDP, thus suggesting that this protein may be involved in the pathogenesis of a variety of neurological disorders. In brains of Huntington-affected patients, pTDP-43 aggregates were shown to co-localize with mutant Huntingtin (mHtt) inclusions. Here, we show that expression of mHtt carrying 80–97 polyglutamines repeats in human cell cultures induces the aggregation and the phosphorylation of endogenous TDP-43, whereas non-pathological Htt with 25 polyglutamines repeats has no effect. Mutant Htt aggregation precedes accumulation of pTDP-43 and pTDP-43 co-localizes with mHtt inclusions reminding what it was previously described in brains of Huntington-affected patients. Detergent-insoluble fractions from cells expressing mHtt and containing mHtt–pTDP-43 co-aggregates can function as seeds for further TDP-43 aggregation in human cell culture. The human cellular prion protein PrPC was previously identified as a negative modulator of mHtt aggregation; here, we show that PrPC-mediated reduction of mHtt aggregation is tightly correlated with a decrease of TDP-43 aggregation and phosphorylation, thus confirming the close relationships between TDP-43 and mHtt.








Similar content being viewed by others
References
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11(4):301–307. https://doi.org/10.1038/nrm2873
Goedert M, Clavaguera F, Tolnay M (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33(7):317–325. https://doi.org/10.1016/j.tins.2010.04.003
Goedert M, Falcon B, Clavaguera F, Tolnay M (2014) Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr Neurol Neurosci Rep 14(11):495. https://doi.org/10.1007/s11910-014-0495-z
Grad LI, Fernando SM, Cashman NR (2015) From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis. Neurobiol Dis 77:257–265. https://doi.org/10.1016/j.nbd.2015.02.009
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501(7465):45–51. https://doi.org/10.1038/nature12481
Ling SC, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79(3):416–438. https://doi.org/10.1016/j.neuron.2013.07.033
Mackenzie IR, Frick P, Neumann M (2014) The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol 127(3):347–357. https://doi.org/10.1007/s00401-013-1232-4
Nonaka T, Hasegawa M (2018) TDP-43 prions. Cold Spring Harb Perspect Med 8(3):a024463. https://doi.org/10.1101/cshperspect.a024463
Prusiner SB (2013) Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47:601–623. https://doi.org/10.1146/annurev-genet-110711-155524
Renner M, Melki R (2014) Protein aggregation and prionopathies. Pathol Biol (Paris) 62(3):162–168. https://doi.org/10.1016/j.patbio.2014.01.003
Sibilla C, Bertolotti A (2017) Prion properties of SOD1 in amyotrophic lateral sclerosis and potential therapy. Cold Spring Harb Perspect Biol 9(10):a024141. https://doi.org/10.1101/cshperspect.a024141
Vilette D, Courte J, Peyrin JM, Coudert L, Schaeffer L, Andreoletti O, Leblanc P (2018) Cellular mechanisms responsible for cell-to-cell spreading of prions. Cell Mol Life Sci. https://doi.org/10.1007/s00018-018-2823-y
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351(3):602–611. https://doi.org/10.1016/j.bbrc.2006.10.093
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. https://doi.org/10.1126/science.1134108
Nishihira Y, Tan CF, Onodera O, Toyoshima Y, Yamada M, Morita T, Nishizawa M, Kakita A, Takahashi H (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116(2):169–182. https://doi.org/10.1007/s00401-008-0385-z
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61(5):435–445. https://doi.org/10.1002/ana.21154
Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VM, Trojanowski JQ (2008) Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115(1):133–145. https://doi.org/10.1007/s00401-007-0257-y
Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K (2007) TDP-43 is deposited in the Guam parkinsonism–dementia complex brains. Brain 130(Pt 5):1386–1394. https://doi.org/10.1093/brain/awm065
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294. https://doi.org/10.1016/j.brainres.2007.09.048
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114(3):221–229. https://doi.org/10.1007/s00401-007-0261-2
Nonaka T, Masuda-Suzukake M, Hasegawa M (2018) Molecular mechanisms of the co-deposition of multiple pathological proteins in neurodegenerative diseases. Neuropathology 38(1):64–71. https://doi.org/10.1111/neup.12427
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141(7):2181–2193. https://doi.org/10.1093/brain/awy146
Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67(6):555–564. https://doi.org/10.1097/NEN.0b013e31817713b5
Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, Lee VM, Kawas CH, Trojanowski JQ (2018) Non-Alzheimer’s contributions to dementia and cognitive resilience in the 90 + study. Acta Neuropathol. https://doi.org/10.1007/s00401-018-1872-5
Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, Sherman MA, Vitzthum CM, Luquette LJ, Yandava CN, Yang P, Chittenden TW, Hatem NE, Ryu SC, Woodworth MB, Park PJ, Walsh CA (2018) Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359(6375):555–559. https://doi.org/10.1126/science.aao4426
Kaushik S, Cuervo AM (2015) Proteostasis and aging. Nat Med 21(12):1406–1415. https://doi.org/10.1038/nm.4001
Huang WJ, Chen WW, Zhang X (2016) Huntington’s disease: molecular basis of pathology and status of current therapeutic approaches. Exp Ther Med 12(4):1951–1956. https://doi.org/10.3892/etm.2016.3566
McColgan P, Tabrizi SJ (2018) Huntington’s disease: a clinical review. Eur J Neurol 25(1):24–34. https://doi.org/10.1111/ene.13413
Gusella JF, MacDonald ME (2000) Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci 1(2):109–115. https://doi.org/10.1038/nrd1077
Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates GP, Lehrach H, Wanker EE (1999) Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci USA 96(8):4604–4609
Rub U, Seidel K, Heinsen H, Vonsattel JP, den Dunnen WF, Korf HW (2016) Huntington’s disease (HD): the neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol 26(6):726–740. https://doi.org/10.1111/bpa.12426
Costanzo M, Zurzolo C (2013) The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem J 452(1):1–17. https://doi.org/10.1042/BJ20121898
Masnata M, Cicchetti F (2017) The evidence for the spread and seeding capacities of the mutant huntingtin protein in in vitro systems and their therapeutic implications. Front Neurosci 11:647. https://doi.org/10.3389/fnins.2017.00647
Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL (2008) Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol 67(12):1159–1165. https://doi.org/10.1097/NEN.0b013e31818e8951
Tada M, Coon EA, Osmand AP, Kirby PA, Martin W, Wieler M, Shiga A, Shirasaki H, Makifuchi T, Yamada M, Kakita A, Nishizawa M, Takahashi H, Paulson HL (2012) Coexistence of Huntington’s disease and amyotrophic lateral sclerosis: a clinicopathologic study. Acta Neuropathol 124(5):749–760. https://doi.org/10.1007/s00401-012-1005-5
St-Amour I, Turgeon A, Goupil C, Planel E, Hebert SS (2018) Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol 135(2):249–265. https://doi.org/10.1007/s00401-017-1786-7
Fuentealba RA, Udan M, Bell S, Wegorzewska I, Shao J, Diamond MI, Weihl CC, Baloh RH (2010) Interaction with polyglutamine aggregates reveals a Q/N-rich domain in TDP-43. J Biol Chem 285(34):26304–26314. https://doi.org/10.1074/jbc.M110.125039
Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO (2016) Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington’s disease: background and evaluation in cell culture. Cell Mol Neurobiol 36(3):459–470. https://doi.org/10.1007/s10571-016-0350-7
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431(7010):805–810. https://doi.org/10.1038/nature02998
Miller J, Arrasate M, Shaby BA, Mitra S, Masliah E, Finkbeiner S (2010) Quantitative relationships between huntingtin levels, polyglutamine length, inclusion body formation, and neuronal death provide novel insight into Huntington’s disease molecular pathogenesis. J Neurosci 30(31):10541–10550. https://doi.org/10.1523/JNEUROSCI.0146-10.2010
Alais S, Soto-Rifo R, Balter V, Gruffat H, Manet E, Schaeffer L, Darlix JL, Cimarelli A, Raposo G, Ohlmann T, Leblanc P (2012) Functional mechanisms of the cellular prion protein (PrP(C)) associated anti-HIV-1 properties. Cell Mol Life Sci 69(8):1331–1352. https://doi.org/10.1007/s00018-011-0879-z
Nonaka T, Arai T, Buratti E, Baralle FE, Akiyama H, Hasegawa M (2009) Phosphorylated and ubiquitinated TDP-43 pathological inclusions in ALS and FTLD-U are recapitulated in SH-SY5Y cells. FEBS Lett 583(2):394–400. https://doi.org/10.1016/j.febslet.2008.12.031
Nonaka T, Kametani F, Arai T, Akiyama H, Hasegawa M (2009) Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet 18(18):3353–3364. https://doi.org/10.1093/hmg/ddp275
Alais S, Simoes S, Baas D, Lehmann S, Raposo G, Darlix JL, Leblanc P (2008) Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol Cell 100(10):603–615. https://doi.org/10.1042/BC20080025
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64(1):60–70. https://doi.org/10.1002/ana.21425
Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M (2008) Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett 582(19):2899–2904. https://doi.org/10.1016/j.febslet.2008.07.027
Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, Hasegawa M (2013) Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 4(1):124–134. https://doi.org/10.1016/j.celrep.2013.06.007
Smethurst P, Newcombe J, Troakes C, Simone R, Chen YR, Patani R, Sidle K (2016) In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol Dis 96:236–247. https://doi.org/10.1016/j.nbd.2016.08.007
Lee KJ, Panzera A, Rogawski D, Greene LE, Eisenberg E (2007) Cellular prion protein (PrPC) protects neuronal cells from the effect of huntingtin aggregation. J Cell Sci 120(Pt 15):2663–2671. https://doi.org/10.1242/jcs.004598
Toyoshima Y, Takahashi H (2014) TDP-43 pathology in polyglutamine diseases: with reference to amyotrophic lateral sclerosis. Neuropathology 34(1):77–82. https://doi.org/10.1111/neup.12053
Shimonaka S, Nonaka T, Suzuki G, Hisanaga S, Hasegawa M (2016) Templated aggregation of TAR DNA-binding protein of 43 kDa (TDP-43) by seeding with TDP-43 peptide fibrils. J Biol Chem 291(17):8896–8907. https://doi.org/10.1074/jbc.M115.713552
Becker LA, Huang B, Bieri G, Ma R, Knowles DA, Jafar-Nejad P, Messing J, Kim HJ, Soriano A, Auburger G, Pulst SM, Taylor JP, Rigo F, Gitler AD (2017) Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 544(7650):367–371. https://doi.org/10.1038/nature22038
Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466(7310):1069–1075. https://doi.org/10.1038/nature09320
Hart MP, Gitler AD (2012) ALS-associated ataxin 2 polyQ expansions enhance stress-induced caspase 3 activation and increase TDP-43 pathological modifications. J Neurosci 32(27):9133–9142. https://doi.org/10.1523/JNEUROSCI.0996-12.2012
Toyoshima Y, Tanaka H, Shimohata M, Kimura K, Morita T, Kakita A, Takahashi H (2011) Spinocerebellar ataxia type 2 (SCA2) is associated with TDP-43 pathology. Acta Neuropathol 122(3):375–378. https://doi.org/10.1007/s00401-011-0862-7
Bonini NM, Gitler AD (2011) Model organisms reveal insight into human neurodegenerative disease: ataxin-2 intermediate-length polyglutamine expansions are a risk factor for ALS. J Mol Neurosci 45(3):676–683. https://doi.org/10.1007/s12031-011-9548-9
Marti E (2016) RNA toxicity induced by expanded CAG repeats in Huntington’s disease. Brain Pathol 26(6):779–786. https://doi.org/10.1111/bpa.12427
Culver BP, DeClercq J, Dolgalev I, Yu MS, Ma B, Heguy A, Tanese N (2016) Huntington’s disease protein huntingtin associates with its own mRNA. J Huntingtons Dis 5(1):39–51. https://doi.org/10.3233/JHD-150177
Nonaka T, Suzuki G, Tanaka Y, Kametani F, Hirai S, Okado H, Miyashita T, Saitoe M, Akiyama H, Masai H, Hasegawa M (2016) Phosphorylation of TAR DNA-binding protein of 43 kDa (TDP-43) by truncated casein kinase 1delta triggers mislocalization and accumulation of TDP-43. J Biol Chem 291(11):5473–5483. https://doi.org/10.1074/jbc.M115.695379
Nonaka T, Masuda-Suzukake M, Hosokawa M, Shimozawa A, Hirai S, Okado H, Hasegawa M (2018) C9ORF72 dipeptide repeat poly-GA inclusions promote: intracellular aggregation of phosphorylated TDP-43. Hum Mol Genet. https://doi.org/10.1093/hmg/ddy174
Porta S, Xu Y, Restrepo CR, Kwong LK, Zhang B, Brown HJ, Lee EB, Trojanowski JQ, Lee VM (2018) Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat Commun 9(1):4220. https://doi.org/10.1038/s41467-018-06548-9
Herrera F, Outeiro TF (2012) alpha-Synuclein modifies huntingtin aggregation in living cells. FEBS Lett 586(1):7–12. https://doi.org/10.1016/j.febslet.2011.11.019
Kayatekin C, Matlack KE, Hesse WR, Guan Y, Chakrabortee S, Russ J, Wanker EE, Shah JV, Lindquist S (2014) Prion-like proteins sequester and suppress the toxicity of huntingtin exon 1. Proc Natl Acad Sci USA 111(33):12085–12090. https://doi.org/10.1073/pnas.1412504111
Pocas GM, Branco-Santos J, Herrera F, Outeiro TF, Domingos PM (2015) alpha-Synuclein modifies mutant huntingtin aggregation and neurotoxicity in Drosophila. Hum Mol Genet 24(7):1898–1907. https://doi.org/10.1093/hmg/ddu606
Tauffenberger A, Chitramuthu BP, Bateman A, Bennett HP, Parker JA (2013) Reduction of polyglutamine toxicity by TDP-43, FUS and progranulin in Huntington’s disease models. Hum Mol Genet 22(4):782–794. https://doi.org/10.1093/hmg/dds485
Katorcha E, Makarava N, Lee YJ, Lindberg I, Monteiro MJ, Kovacs GG, Baskakov IV (2017) Cross-seeding of prions by aggregated alpha-synuclein leads to transmissible spongiform encephalopathy. PLoS Pathog 13(8):e1006563. https://doi.org/10.1371/journal.ppat.1006563
Derkatch IL, Uptain SM, Outeiro TF, Krishnan R, Lindquist SL, Liebman SW (2004) Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc Natl Acad Sci USA 101(35):12934–12939. https://doi.org/10.1073/pnas.0404968101
Tomas-Zapico C, Diez-Zaera M, Ferrer I, Gomez-Ramos P, Moran MA, Miras-Portugal MT, Diaz-Hernandez M, Lucas JJ (2012) alpha-Synuclein accumulates in huntingtin inclusions but forms independent filaments and its deficiency attenuates early phenotype in a mouse model of Huntington’s disease. Hum Mol Genet 21(3):495–510. https://doi.org/10.1093/hmg/ddr507
Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G (2012) TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res 1462:16–25. https://doi.org/10.1016/j.brainres.2012.02.032
Acknowledgements
This work was supported by the CNRS and the INSERM (PL), and Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) (JP26117005) from MEXT (to M.H.), a Grant-in-Aid for Scientific Research on Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) (JP14533254) from AMED (to M.H.), JST CREST Grant number JP18071300, Japan, and a Grant from Brain Science Foundation (to T.N.). PL received the support of the Association pour la recherche sur la SLA (ARSLA), the NeuroDis Fundation and the AFM (AFM-MyoNeuralp). LC received for his PhD an ARC2 “Qualité de vie et Viellissement” Grant from the région Auvergne Rhône-Alpes. We thank Didier Vilette (ENVT–Toulouse) for carefully reading the manuscript and helpful discussions. We acknowledge the LYMIC-PLATIM and the CIQLE microscope platforms at ENS Lyon (SFR Biosciences Gerland–Lyon Sud UMS3444/US8, France) and at the Faculté de médecine Rockefeller (Lyon Est, France) respectively.
Author information
Authors and Affiliations
Contributions
TN, LC and PL conducted the experiments. All the authors interpreted the data and corrected the paper. PL wrote the manuscript.
Corresponding author
Ethics declarations
Ethical standards
Experiments described in this manuscript comply with the current laws of the country.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coudert, L., Nonaka, T., Bernard, E. et al. Phosphorylated and aggregated TDP-43 with seeding properties are induced upon mutant Huntingtin (mHtt) polyglutamine expression in human cellular models. Cell. Mol. Life Sci. 76, 2615–2632 (2019). https://doi.org/10.1007/s00018-019-03059-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03059-8