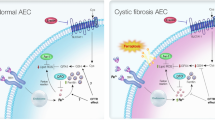
Abstract
Mutations in the gene encoding the CFTR chloride channel produce cystic fibrosis (CF). CF patients are more susceptible to bacterial infections in lungs. The most accepted hypothesis sustains that a reduction in the airway surface liquid (ASL) volume favor infections. Alternatively, it was postulated that a reduced HCO3− transport through CFTR leads to a decreased ASL pH, favoring bacterial colonization. The issue is controversial, since recent data from cultured primary cells and CF children showed normal pH values in the ASL. We have reported previously a decreased mitochondrial Complex I (mCx-I) activity in cultured cells with impaired CFTR activity. Thus, we hypothesized that the reduced mCx-I activity could lead to increased lactic acid production (Warburg-like effect) and reduced extracellular pH (pHe). In agreement with this idea, we report here that cells with impaired CFTR function (intestinal Caco-2/pRS26, transfected with an shRNA-CFTR, and lung IB3-1 CF cells) have a decreased pHe. These cells showed increased lactate dehydrogenase (LDH) activity, LDH-A expression, and lactate secretion. Similar effects were reproduced in control cells stimulated with recombinant IL-1β. The c-Src and JNK inhibitors PP2 and SP600125 were able to increase the pHe, although the differences between control and CFTR-impaired cells were not fully compensated. Noteworthy, the LDH inhibitor oxamate completely restored the pHe of the intestinal Caco-2/pRS26 cells and have a significant effect in lung IB3-1 cells; therefore, an increased lactic acid secretion seems to be the key factor that determine a reduced pHe in these epithelial cells.








Similar content being viewed by others
Abbreviations
- ASL:
-
Airway surface liquid
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CFTR-KD:
-
CFTR knockdown
- DMSO:
-
Dimethyl sulfoxide
- IL-1β:
-
Interleukin-1β
- JNK:
-
JUN N-terminal kinase
- LDH:
-
Lactate dehydrogenase
- mCx-I:
-
Mitochondrial Complex I
- pHe:
-
Extracellular pH
- shRNA:
-
Short hairpin RNA
References
Riordan JR et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245(4922):1066–1073
Sheppard DN, Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79(1 Suppl):S23–S45
Rommens JM et al (1991) cAMP-inducible chloride conductance in mouse fibroblast lines stably expressing the human cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 88(17):7500–7504
Althaus M (2013) ENaC inhibitors and airway re-hydration in cystic fibrosis: state of the art. Curr Mol Pharmacol 6(1):3–12
Chen JH et al (2010) Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143(6):911–923
Rogers CS et al (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321(5897):1837–1841
Pezzulo AA et al (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487(7405):109–113
McShane D et al (2003) Airway surface pH in subjects with cystic fibrosis. Eur Respir J 21(1):37–42
Schultz A et al (2017) Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun 8(1):1409
Massip-Copiz MM, Santa-Coloma TA (2018) Extracellular pH and lung infections in cystic fibrosis. Eur J Cell Biol 97:402–410
Figueira MF, Webster MJ, Tarran R (2018) CrossTalk proposal: mucosal acidification drives early progressive lung disease in cystic fibrosis. J Physiol 596(16):3433–3437
González Guerrico AM et al (1999) Abstract M238 [El gen c-src es un posible marcador funcional del canal de cloruro afectado en fibrosis quística: CFTR]. In: 35th Annual meeting of the argentine society for biochemistry and molecular biology research (SAIB), November 9-12, Mendoza, Argentina (abstracts book)
Cafferata EG et al (1995) Abstract M99 [Identificación mediante “differential display” de genes específicamente regulados por diferentes factores que afectan la expresión del CFTR (canal de cloro afectado en Fibrosis Quística)] Abstracts of the 31th annual meeting of the Argentine Society for biochemistry and molecular biology research (SAIB), 15–18 November, Villa Giardino, Córdoba (abstracts book)
Gonzalez-Guerrico AM et al (2002) Tyrosine kinase c-Src constitutes a bridge between cystic fibrosis transmembrane regulator channel failure and MUC1 overexpression in cystic fibrosis. J Biol Chem 277(19):17239–17247
Estell K et al (2003) Plasma membrane CFTR regulates RANTES expression via its C-terminal PDZ-interacting motif. Mol Cell Biol 23(2):594–606
Valdivieso AG et al (2007) The expression of the mitochondrial gene MT-ND4 is downregulated in cystic fibrosis. Biochem Biophys Res Commun 356(3):805–809
Taminelli GL et al (2008) CISD1 codifies a mitochondrial protein upregulated by the CFTR channel. Biochem Biophys Res Commun 365(4):856–862
Hofhaus G, Attardi G (1993) Lack of assembly of mitochondrial DNA-encoded subunits of respiratory NADH dehydrogenase and loss of enzyme activity in a human cell mutant lacking the mitochondrial ND4 gene product. EMBO J 12(8):3043–3048
Valdivieso AG, Santa-Coloma TA (2013) CFTR activity and mitochondrial function. Redox Biol 1(1):190–202
Valdivieso AG et al (2012) The mitochondrial complex I activity is reduced in cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS One 7(11):e48059
Clauzure M et al (2014) Disruption of interleukin-1beta autocrine signaling rescues complex I activity and improves ROS levels in immortalized epithelial cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS One 9(6):e99257
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Alfarouk KO et al (2014) Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 1(12):777–802
Zhou M et al (2010) Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer 9:33
Robinson BH (2006) Lactic acidemia and mitochondrial disease. Mol Genet Metab 89(1–2):3–13
Massip-Copiz MM et al (2017) CFTR impairment upregulates c-Src activity through IL-1beta autocrine signaling. Arch Biochem Biophys 616:1–12
Zeitlin PL et al (1991) A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol 4(4):313–319
Bargon J et al (1992) Expression of the cystic fibrosis transmembrane conductance regulator gene can be regulated by protein kinase C. J Biol Chem 267(23):16056–16060
Shoshani T et al (1992) Association of a nonsense mutation (W1282X), the most common mutation in the Ashkenazi Jewish cystic fibrosis patients in Israel, with presentation of severe disease. Am J Hum Genet 50(1):222–228
Zeitlin PL et al (1992) CFTR protein expression in primary and cultured epithelia. Proc Natl Acad Sci USA 89(1):344–347
Flotte TR et al (1993) Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA 90(22):10613–10617
Flotte TR et al (1993) Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 268(5):3781–3790
Purkey HE et al (2016) Cell active hydroxylactam inhibitors of human lactate dehydrogenase with oral bioavailability in mice. ACS Med Chem Lett 7(10):896–901
Brighenti E et al (2017) The inhibition of lactate dehydrogenase A hinders the transcription of histone 2B gene independently from the block of aerobic glycolysis. Biochem Biophys Res Commun 485(4):742–745
Chen EP et al (1989) Inactivation of lactate dehydrogenase by UV radiation in the 300 nm wavelength region. Radiat Environ Biophys 28(3):185–191
Schmid I, Uittenbogaart CH, Giorgi JV (1991) A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry 12(3):279–285
Brady KD et al (1994) Relationships between amplitudes and kinetics of rapid cytosolic free calcium fluctuations in GH4C1 rat pituitary cells: roles for diffusion and calcium-induced calcium release. Biophys J 66(5):1697–1705
Levene H (1960) Robust tests for equality of variances in contributions to probability and statistics: essays in honor of Harold Hotelling, I. Olkin, et al., editors. Stanford University Press, Palo Alto, pp 278–292
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52(3–4):591–611
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18(1):50–60
Student (Gossett W.S.) (1907) On the error of counting with a haemacytometer. Biometrika 5(3):351–360
Counillon L et al (2016) Na(+)/H(+) antiporter (NHE1) and lactate/H(+) symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim Biophys Acta 1863(10):2465–2480
Halestrap AP (2013) The SLC16 gene family—structure, role and regulation in health and disease. Mol Aspects Med 34(2–3):337–349
Halestrap AP (2013) Monocarboxylic acid transport. Compr Physiol 3(4):1611–1643
Massip-Copiz M et al (2018) Epiregulin (EREG) is upregulated through an IL-1beta autocrine loop in Caco-2 epithelial cells with reduced CFTR function. J Cell Biochem 119(3):2911–2922
Valdivieso AG et al (2017) CFTR modulates RPS27 gene expression using chloride anion as signaling effector. Arch Biochem Biophys 633:103–109
Clauzure M et al (2017) Intracellular chloride concentration changes modulate IL-1beta expression and secretion in human bronchial epithelial cultured cells. J Cell Biochem 118(8):2131–2140
Valdivieso AG et al (2016) The chloride anion acts as a second messenger in mammalian cells—modifying the expression of specific genes. Cell Physiol Biochem 38(1):49–64
Bardon A (1987) Cystic fibrosis. Carbohydrate metabolism in CF and in animal models for CF. Acta Paediatr Scand Suppl 332:1–30
Bardon A, Ceder O, Kollberg H (1986) Increased activity of four glycolytic enzymes in cultured fibroblasts from cystic fibrosis patients. Res Commun Chem Pathol Pharmacol 51(3):405–408
Shapiro BL (1988) Mitochondrial dysfunction, energy expenditure, and cystic fibrosis. Lancet 2(8605):289
Shapiro BL, Feigal RJ, Lam LF (1979) Mitrochondrial NADH dehydrogenase in cystic fibrosis. Proc Natl Acad Sci USA 76(6):2979–2983
Quinton PM (2007) Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 22:212–225
Abcouwer SF et al (2008) Effect of IL-1beta on survival and energy metabolism of R28 and RGC-5 retinal neurons. Invest Ophthalmol Vis Sci 49(12):5581–5592
Manerba M et al (2017) Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Eur J Pharm Sci 96:37–44
Wetmore DR et al (2010) Metabolomic profiling reveals biochemical pathways and biomarkers associated with pathogenesis in cystic fibrosis cells. J Biol Chem 285(40):30516–30522
Bensel T et al (2011) Lactate in cystic fibrosis sputum. J Cyst Fibros 10(1):37–44
Kottmann RM et al (2012) Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med 186(8):740–751
Ye X, Lotan R (2008) Potential misinterpretation of data on differential gene expression in normal and malignant cells in vitro. Brief Funct Genom Proteom 7(4):322–326
Bijman J, Quinton PM (1987) Lactate and bicarbonate uptake in the sweat duct of cystic fibrosis and normal subjects. Pediatr Res 21(1):79–82
Ishiguro H et al (2012) Physiology and pathophysiology of bicarbonate secretion by pancreatic duct epithelium. Nagoya J Med Sci 74(1–2):1–18
Garnett JP et al (2016) Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H + secretion. Sci Rep 6:37955
Lin YC et al (2018) The pseudomonas aeruginosa complement of lactate dehydrogenases enables use of d- and l-lactate and metabolic cross-feeding. MBio 9(5):e00961-18
Silva IN et al (2017) Regulator LdhR and d-lactate dehydrogenase LdhA of burkholderia multivorans play roles in carbon overflow and in planktonic cellular aggregate formation. Appl Environ Microbiol 83(19):e01343-17
Miskimins WK et al (2014) Synergistic anti-cancer effect of phenformin and oxamate. PLoS One 9(1):e85576
Zhao Z et al (2015) Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett 358(1):17–26
Massip Copiz MM, Santa Coloma TA (2016) c- Src and its role in cystic fibrosis. Eur J Cell Biol 95(10):401–413
Acknowledgements
We thank Professor Diego Battiato and Romina D’Agostino for administrative assistance, and María de los Angeles Aguilar for technical assistance. We also thank Dr. Lutz Birnbaumer for his continuous support to our work and very valuable criticisms. This work was supported by National Agency for the Promotion of Science and Technology (ANPCYT) [grant numbers PICT 2012-1278 to TASC and PICT-2015-1031 to AGV]; National Scientific and Technical Research Council of Argentina (CONICET) [grants PIP-2016 112201-501002-27 and PUE-2016 22920160100129CO to TASC]; and Pontifical Catholic University of Argentina (UCA) to TASC. Fellowships from CONICET to MMC, CM, and MC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valdivieso, Á.G., Clauzure, M., Massip-Copiz, M.M. et al. Impairment of CFTR activity in cultured epithelial cells upregulates the expression and activity of LDH resulting in lactic acid hypersecretion. Cell. Mol. Life Sci. 76, 1579–1593 (2019). https://doi.org/10.1007/s00018-018-3001-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-3001-y