Abstract
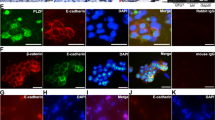
The self-renewal capacity of the stem cell pool determines tissue function and health. Cadherin-22 (Cdh22), a member of the cadherin superfamily, has two splicing patterns in rats, and the short type that lacks a catenin binding domain is closely related to spermatogonial stem cell self-renewal. Previously, we reported that CDH22 was highly expressed in mouse ovary germ cells, especially in female germ line stem cells (FGSCs). However, its underlying function in FGSCs is still not clear. Here, we found that Cdh22 encodes only one type of protein product in mice and demonstrated that CDH22 was required for FGSC self-renewal. In addition, JAK2 and β-catenin were found to interact with CDH22 and be involved in CDH22 signaling in mouse FGSCs. Moreover, extrinsic CDH22 was identified as a potential molecule that participates in FGSC adhesion and is pivotal for FGSC maintenance and self-renewal. These results reveal that CDH22 functions as an essential molecule in FGSC maintenance and self-renewal via different mechanisms, including interaction with the JAK-STAT signaling pathway and β-catenin.






Similar content being viewed by others
Abbreviations
- BrdU:
-
5-Bromo-2′-deoxyuridine
- CDH22:
-
Cadherin-22
- FGSCs:
-
Female germ line stem cells
- DMSO:
-
Dimethyl sulphoxide
- JAK–STAT:
-
Janus kinase–signal transducer and activator of transcription
- PCNA:
-
Proliferating cell nuclear antigen
References
Zuckerman S (1951) The number of oocytes in the mature ovary. Horm Res 6:63–108
Borum K (1961) Oogenesis in the mouse : a study of the meiotic prophase. Exp Cell Res 24(3):495–507
Peters H (1970) Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc Lond 259(828):91–101
Mclaren A (1984) Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol 38(38):7
Faddy MJ, Jones EC, Edwards RG (1976) An analytical model for ovarian follicle dynamics. J Exp Zool 197(2):173
Perez GI et al (1999) Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21(2):200–203
Faddy MJ (2000) Follicle dynamics during ovarian ageing. Mol Cell Endocrinol 163(1–2):43–48
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2(11):838–848
Johnson J et al (2004) Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428(6979):145–150
Johnson J et al (2005) Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122(2):303–315
Hu Y et al (2012) GSK3 inhibitor-BIO regulates proliferation of female germline stem cells from the postnatal mouse ovary. Cell Prolif 45(4):287–298
Bai Y et al (2013) Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif 46(5):516–528
Nakamura S et al (2010) Identification of germline stem cells in the ovary of the teleost medaka. Science 328(5985):1561–1563
Zou K et al (2009) Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 11(5):631–636
White YAR et al (2012) Oocyte formation by mitotically-active germ cells purified from ovaries of reproductive age women. Nat Med 18(3):413–421
Guo K et al (2016) Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod 22(5):316–328
Zhang H et al (2012) Experimental evidence showing that no mitotically active female germ line progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA 109(31):12580
Lei L, Spradling AC (2013) Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA 110(21):8585
Woods DC, Tilly JL (2015) Reply to Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med 21(10):1116–1118
Zarate-Garcia L et al (2016) FACS-sorted putative oogonial stem cells from the ovary are neither DDX4-positive nor germ cells. Sci Rep 6:27991
Li X, Ao J, Wu J (2017) Systematic identification and comparison of expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in mouse germline stem cells. Oncotarget 8(16):26573–26590
Wu C et al (2017) Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol Ther 25(6):1408–1419
Ding X et al (2016) Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep 6:28218
Zhang Y et al (2011) Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol 3(2):132–141
Zou K et al (2011) Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev 20(12):2197–2204
Woods DC, Tilly JL (2013) Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc 8(5):966–988
Wang H et al (2014) Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol 6(2):164–171
Xie W, Wang H, Wu J (2014) Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep 4:5580
Zhou L et al (2014) Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod 20(3):271–281
Khosravifarsani S et al (2015) Isolation and enrichment of mouse female germ line stem cells. Cell J 16(4):406–415
Park ES, Tilly JL (2015) Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod 21(1):58
Lu Z et al (2016) Improvement in isolation and identification of mouse oogonial stem cells. Stem Cell Int 2016(19):1–10
Zhang C, Wu J (2016) Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod 22(7):457
Pacchiarotti J et al (2010) Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 79(3):159–170
Park ES, Woods DC, Tilly JL (2013) Bone morphogenetic protein 4 (BMP4) promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril 100(5):1468–1475
Song X, Xie T (2002) DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA 99(23):14813–14818
Tokuda M et al (2007) CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod 76(1):130–141
Baronsky T et al (2016) Reduction in E-cadherin expression fosters migration of Xenopus laevis primordial germ cells. Integr Biol 8(3):349–358
Sugimoto K et al (1996) Molecular cloning and characterization of a newly identified member of the cadherin family, PB-cadherin. J Biol Chem 271(19):11548–11556
Wu J et al (2003) Expression of a novel factor, short-type PB-cadherin, in Sertoli cells and spermatogenic stem cells of the neonatal rat testis. J Endocrinol 176(3):381
Wu J, Jester W, Orth J (2005) Short-type PB-cadherin promotes survival of gonocytes and activates JAK-STAT signalling. Dev Biol 284(2):437–450
Wu J et al (2008) Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal 20(6):1052
Kitajima K, Koshimizu U, Nakamura T (1999) Expression of a novel type of classic cadherin, PB-cadherin in developing brain and limb buds. Dev Dyn 215(3):206–214
Hirano T, Nakajima K, Hibi M (1997) Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev 8(4):241
Kanatsushinohara M et al (2008) Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell 3(5):533
Kiger AA et al (2001) Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294(5551):2542
Acknowledgements
This work was supported by National Natural Science Foundation of China (81200472) and Fundamental Research Funds for the Central Universities in China (KYTZ201602).
Author information
Authors and Affiliations
Contributions
XZ, collection and/or assembly of data, data analysis and interpretation. YY, collection and/or assembly of data, data analysis and interpretation. QX, collection and/or assembly of data, data analysis and interpretation. HS, collection and/or assembly of data, and data analysis. RW, collection and/or assembly of data, and data analysis. JW, collection and/or assembly of data, and data analysis. KZ, conception and design, financial support, manuscript writing, and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, Y., Xia, Q. et al. Cadherin 22 participates in the self-renewal of mouse female germ line stem cells via interaction with JAK2 and β-catenin. Cell. Mol. Life Sci. 75, 1241–1253 (2018). https://doi.org/10.1007/s00018-017-2689-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2689-4