Abstract
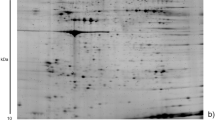
Colorectal cancer (CRC) is one of the most frequent malignancies in the Western world. Early tumor detection and intervention are important determinants on CRC patient survival. During early tumor proliferation, dissemination and angiogenesis, platelets store and segregate proteins actively and selectively. Hence, the platelet proteome is a potential source of biomarkers denoting early malignancy. By comparing protein profiles of platelets between healthy volunteers (n = 12) and patients with early- (n = 7) and late-stage (n = 5) CRCs using multiplex fluorescence two-dimensional gel electrophoresis (2D-DIGE), we aimed at identifying differentially regulated proteins within platelets. By inter-group comparisons, 94 differentially expressed protein spots were detected (p < 0.05) between healthy controls and patients with early- and late-stage CRCs and revealed distinct separations between all three groups in principal component analyses. 54 proteins of interest were identified by mass spectrometry and resulted in high-ranked Ingenuity Pathway Analysis networks associated with Cellular function and maintenance, Cellular assembly and organization, Developmental disorder and Organismal injury and abnormalities (p < 0.0001 to p = 0.0495). Target proteins were validated by multiplex fluorescence-based Western blot analyses using an additional, independent cohort of platelet protein samples [healthy controls (n = 15), early-stage CRCs (n = 15), late-stage CRCs (n = 15)]. Two proteins—clusterin and glutathione synthetase (GSH-S)—featured high impact and were subsequently validated in this independent clinical cohort distinguishing healthy controls from patients with early- and late-stage CRCs. Thus, the potential of clusterin and GSH-S as platelet biomarkers for early detection of CRC could improve existing screening modalities in clinical application and should be confirmed in a prospective multicenter trial.




Similar content being viewed by others
Abbreviations
- 2D-DIGE:
-
Two-dimensional multiplex fluorescence gel electrophoresis
- BHT:
-
Butylated hydroxytoluene
- CLU:
-
Clusterin
- CRC:
-
Colorectal cancer
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetate
- EMT:
-
Epithelial-to-mesenchymal transition
- FC:
-
Fold change
- GSH-S:
-
Glutathione synthetase
- IEF:
-
Isoelectric focusing
- IPA:
-
Ingenuity Pathway Analysis
- IPG:
-
Immobilized pH gradient
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization time of flight mass spectrometry
- PBS:
-
Phosphate-buffered saline
- PCA:
-
Principal component analysis
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PTMs:
-
Posttranslational modifications
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBS:
-
Tris-buffered saline
- UICC:
-
International Union Against Cancer
References
Schiess R, Wollscheid B, Aebersold R (2009) Targeted proteomic strategy for clinical biomarker discovery. Mol Oncol 3(1):33–44. doi:10.1016/j.molonc.2008.12.001
Anderson NL, Anderson NG (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1(11):845–867
Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD (2003) Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics 2(10):1096–1103. doi:10.1074/mcp.M300031-MCP200
Jacobs JM, Adkins JN, Qian WJ, Liu T, Shen Y, Camp DG 2nd, Smith RD (2005) Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res 4(4):1073–1085. doi:10.1021/pr0500657
Sierko E, Wojtukiewicz MZ (2004) Platelets and angiogenesis in malignancy. Semin Thromb Hemost 30(1):95–108. doi:10.1055/s-2004-822974
Goubran HA, Stakiw J, Radosevic M, Burnouf T (2014) Platelets effects on tumor growth. Semin Oncol 41(3):359–369. doi:10.1053/j.seminoncol.2014.04.006
Sierko E, Wojtukiewicz MZ (2007) Inhibition of platelet function: does it offer a chance of better cancer progression control? Semin Thromb Hemost 33(7):712–721. doi:10.1055/s-2007-991540
Bambace NM, Holmes CE (2011) The platelet contribution to cancer progression. J Thromb Haemost 9(2):237–249. doi:10.1111/j.1538-7836.2010.04131.x
Coupland LA, Parish CR (2014) Platelets, selectins, and the control of tumor metastasis. Semin Oncol 41(3):422–434. doi:10.1053/j.seminoncol.2014.04.003
Bastida E, Almirall L, Ordinas A (1987) Tumor-cell-induced platelet aggregation is a glycoprotein-dependent and lipoxygenase-associated process. Int J Cancer 39(6):760–763
Honn KV, Tang DG, Chen YQ (1992) Platelets and cancer metastasis: more than an epiphenomenon. Semin Thromb Hemost 18(4):392–415. doi:10.1055/s-2007-1002578
Burkhart JM, Gambaryan S, Watson SP, Jurk K, Walter U, Sickmann A, Heemskerk JW, Zahedi RP (2014) What can proteomics tell us about platelets? Circ Res 114(7):1204–1219. doi:10.1161/CIRCRESAHA.114.301598
Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP (2012) The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 120(15):e73–e82. doi:10.1182/blood-2012-04-416594
Blann AD, Gurney D, Wadley M, Bareford D, Stonelake P, Lip GY (2001) Increased soluble P-selectin in patients with haematological and breast cancer: a comparison with fibrinogen, plasminogen activator inhibitor and von Willebrand factor. Blood Coagul Fibrinolysis 12(1):43–50
Caine GJ, Lip GY, Stonelake PS, Ryan P, Blann AD (2004) Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost 92(1):185–190. doi:10.1267/THRO04070185
Reed GL, Fitzgerald ML, Polgar J (2000) Molecular mechanisms of platelet exocytosis: insights into the “secrete” life of thrombocytes. Blood 96(10):3334–3342
Rendu F, Brohard-Bohn B (2001) The platelet release reaction: granules’ constituents, secretion and functions. Platelets 12(5):261–273. doi:10.1080/09537100120068170
Zufferey A, Fontana P, Reny JL, Nolli S, Sanchez JC (2012) Platelet proteomics. Mass Spectrom Rev 31(2):331–351. doi:10.1002/mas.20345
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–590. doi:10.1016/j.ccr.2011.09.009
Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB (2004) Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103(6):2096–2104. doi:10.1182/blood-2003-08-2804
Sabrkhany S, Griffioen AW, Oude Egbrink MG (2011) The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta 1815(2):189–196. doi:10.1016/j.bbcan.2010.12.001
Patrignani P, Patrono C (2016) Aspirin and Cancer. J Am Coll Cardiol 68(9):967–976. doi:10.1016/j.jacc.2016.05.083
Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M (2010) The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010:215158. doi:10.1155/2010/215158
Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, John CM, Suess B, Sgambato A, Steinhilber D, Patrignani P (2013) Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol 84(1):25–40. doi:10.1124/mol.113.084988
Dovizio M, Tacconelli S, Ricciotti E, Bruno A, Maier TJ, Anzellotti P, Di Francesco L, Sala P, Signoroni S, Bertario L, Dixon DA, Lawson JA, Steinhilber D, FitzGerald GA, Patrignani P (2012) Effects of celecoxib on prostanoid biosynthesis and circulating angiogenesis proteins in familial adenomatous polyposis. J Pharmacol Exp Ther 341(1):242–250. doi:10.1124/jpet.111.190785
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917. doi:10.1002/ijc.25516
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. doi:10.3322/caac.21262
Arvelo F, Sojo F, Cotte C (2015) Biology of colorectal cancer. Ecancermedicalscience 9:520. doi:10.3332/ecancer.2015.520
Connell W (2004) PRO: endoscopic surveillance minimizes the risk of cancer. Am J Gastroenterol 99(9):1631–1633. doi:10.1111/j.1572-0241.2004.40829.x
Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, Grande R, Alberti S, Arena V, Cirillo M, Patrono C, FitzGerald GA, Steinhilber D, Sgambato A, Patrignani P (2016) Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget 7(22):32462–32477. doi:10.18632/oncotarget.8655
Ferroni P, Riondino S, Vazzana N, Santoro N, Guadagni F, Davi G (2012) Biomarkers of platelet activation in acute coronary syndromes. Thromb Haemost 108(6):1109–1123. doi:10.1160/TH12-08-0550
Strohkamp S, Gemoll T, Habermann JK (2016) Possibilities and limitations of 2DE-based analyses for identifying low-abundant tumor markers in human serum and plasma. Proteomics 16(19):2519–2532. doi:10.1002/pmic.201600154
Gemoll T, Epping F, Heinrich L, Fritzsche B, Roblick UJ, Szymczak S, Hartwig S, Depping R, Bruch HP, Thorns C, Lehr S, Paech A, Habermann JK (2015) Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget 6(18):16517–16526. doi:10.18632/oncotarget.4140
Hagner-McWhirter A, Laurin Y, Larsson A, Bjerneld EJ, Ronn O (2015) Cy5 total protein normalization in Western blot analysis. Anal Biochem 486:54–61. doi:10.1016/j.ab.2015.06.017
Jones SE, Jomary C (2002) Clusterin. The International Journal of Biochemistry & Cell Biology 34(5):427–431
Rodriguez-Pineiro AM, Garcia-Lorenzo A, Blanco-Prieto S, Alvarez-Chaver P, Rodriguez-Berrocal FJ, Cadena MP, Martinez-Zorzano VS (2012) Secreted Clusterin in colon tumor cell models and its potential as diagnostic marker for colorectal cancer. Cancer Invest 30(1):72–78. doi:10.3109/07357907.2011.630051
Pucci S, Bonanno E, Pichiorri F, Mazzarelli P, Spagnoli LG (2004) The expression and the nuclear activity of the caretaker gene ku86 are modulated by somatostatin. Eur J Histochem 48(2):103–110
Pucci S, Bonanno E, Sesti F, Mazzarelli P, Mauriello A, Ricci F, Zoccai GB, Rulli F, Galata G, Spagnoli LG (2009) Clusterin in stool: a new biomarker for colon cancer screening? Am J Gastroenterol 104(11):2807–2815. doi:10.1038/ajg.2009.412
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Kim AD, Zhang R, Han X, Kang KA, Piao MJ, Maeng YH, Chang WY, Hyun JW (2015) Involvement of glutathione and glutathione metabolizing enzymes in human colorectal cancer cell lines and tissues. Mol Med Rep 12(3):4314–4319. doi:10.3892/mmr.2015.3902
Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 89(7):3070–3074
Lu LI, Fu NI, Luo XU, Li XY, Li XP (2015) Overexpression of cofilin 1 in prostate cancer and the corresponding clinical implications. Oncol Lett 9(6):2757–2761. doi:10.3892/ol.2015.3133
Chang CY, Leu JD, Lee YJ (2015) The actin depolymerizing factor (ADF)/cofilin signaling pathway and DNA damage responses in cancer. Int J Mol Sci 16(2):4095–4120. doi:10.3390/ijms16024095
Zhu B, Fukada K, Zhu H, Kyprianou N (2006) Prohibitin and cofilin are intracellular effectors of transforming growth factor beta signaling in human prostate cancer cells. Cancer Res 66(17):8640–8647. doi:10.1158/0008-5472.CAN-06-1443
Zhou J, Wang Y, Fei J, Zhang W (2012) Expression of cofilin 1 is positively correlated with the differentiation of human epithelial ovarian cancer. Oncol Lett 4(6):1187–1190. doi:10.3892/ol.2012.897
Hembrough T, Thyparambil S, Liao WL, Darfler MM, Abdo J, Bengali KM, Hewitt SM, Bender RA, Krizman DB, Burrows J (2013) Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J Mol Diagn 15(4):454–465. doi:10.1016/j.jmoldx.2013.03.002
Nishimura S, Tsuda H, Kataoka F, Arao T, Nomura H, Chiyoda T, Susumu N, Nishio K, Aoki D (2011) Overexpression of cofilin 1 can predict progression-free survival in patients with epithelial ovarian cancer receiving standard therapy. Hum Pathol 42(4):516–521. doi:10.1016/j.humpath.2010.07.019
Chung H, Kim B, Jung SH, Won KJ, Jiang X, Lee CK, Lim SD, Yang SK, Song KH, Kim HS (2013) Does phosphorylation of cofilin affect the progression of human bladder cancer? BMC Cancer 13:45. doi:10.1186/1471-2407-13-45
Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, Zhang X, Oka M, Nakamura K (2011) Differential expression of up-regulated cofilin-1 and down-regulated cofilin-2 characteristic of pancreatic cancer tissues. Oncol Rep 26(6):1595–1599. doi:10.3892/or.2011.1447
Castro MA, Dal-Pizzol F, Zdanov S, Soares M, Muller CB, Lopes FM, Zanotto-Filho A, da Cruz Fernandes M, Moreira JC, Shacter E, Klamt F (2010) CFL1 expression levels as a prognostic and drug resistance marker in nonsmall cell lung cancer. Cancer 116(15):3645–3655. doi:10.1002/cncr.25125
Tonus C, Neupert G, Sellinger M (2006) Colorectal cancer screening by non-invasive metabolic biomarker fecal tumor M2-PK. World J Gastroenterol 12(43):7007–7011
Deutekom M, van Rossum LG, van Rijn AF, Laheij RJ, Fockens P, Bossuyt PM, Dekker E, Jansen JB (2010) Comparison of guaiac and immunological fecal occult blood tests in colorectal cancer screening: the patient perspective. Scand J Gastroenterol 45(11):1345–1349. doi:10.3109/00365521.2010.497937
van Rossum LG, van Rijn AF, Verbeek AL, van Oijen MG, Laheij RJ, Fockens P, Jansen JB, Adang EM, Dekker E (2011) Colorectal cancer screening comparing no screening, immunochemical and guaiac fecal occult blood tests: a cost-effectiveness analysis. Int J Cancer 128(8):1908–1917. doi:10.1002/ijc.25530
Acknowledgements
Grants from the Werner and Clara Kreitz Foundation and the Ad Infinitum Foundation are gratefully acknowledged. This study was performed in connection with the Surgical Center for Translational Oncology—Lübeck (SCTO-L) and the North German Tumorbank of Colorectal Cancer (ColoNet), the latter being generously supported by the German Cancer Aid Foundation (DKH e.V. #108446).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
This study was approved by the local Ethics Committee of the University of Lübeck (#07-124). Blood samples of CRC patients and healthy volunteers were processed after informed consent and stored at the Interdisciplinary Centrum for Biobanking-Lübeck (ICB-L). The experiments comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2017_2631_MOESM1_ESM.pdf
Supplementary Fig. 1: Workflow of the study design. Supplementary Fig. 2 Preliminary in vitro platelet activation experiment. (A) Using a standard operating protocol, platelets were isolated from EDTA anticoagulated venous blood as a homogenous suspension. (B) After addition of 5 µg/ml collagen, 0.5 U/ml thrombin and 13.8 mmol/l CaCl2, artificially platelet activation was visible as white aggregates. Supplementary Fig. 3 Supervised PCA plots between (A) healthy controls (n = 12, pink) vs. late-stage CRCs (n = 5, purple) based on 44 significant spots, of (B) early-stage CRCs (n = 7, blue) vs. late-stage CRCs (n = 5, purple) based on 36 protein spots with significant changes (t test, p < 0.05) and between (C) healthy controls (n = 12, pink) vs. early-stage CRCs (n = 7, blue) vs. late-stage CRCs (n = 5, purple) based on 39 differentially expressed spots (1-way ANOVA, p < 0.05). Colored dots represent platelet samples from each group. Supplementary Fig. 4 2D-DIGE protein expression profiles of Clusterin (A), GSH-S (B) and Cofilin-1 (C) between healthy controls, early-stage CRCs and late-stage CRCs (PDF 1019 kb)
18_2017_2631_MOESM2_ESM.pdf
Supplementary Table 1 Clinical data of the evaluated patient cohort of the 2-D DIGE experiment. Supplementary Table 2 Clinical data of the evaluated patient cohort of the Western blot experiment. Supplementary Table 3 Summary of identified platelet proteins using MALDI-TOF/TOF–MS. Platelet protein identifications of 71 spots with significant differences in 2D-DIGE (t test, p < 0.05) using MALDI-TOF/TOF–MS and Mascot database. Mascot Scores marked with an asterisks (*) indicate MS data. Several spots are isoforms of the same protein. Arrows indicate significant protein down- (↓) or upregulation (↑) in CRC cancer samples compared to healthy controls (t test, p < 0.05 and 1-way ANOVA, p < 0.05). Supplementary Table 4 Summary of platelet protein pathway analyses of direct relationships using IPA. Supplemental Table 5 Diagnostic parameters for the Healthy volunteers vs. Early CRC comparison (top) and associated ROC-curve (bottom) for Clusterin (CLU). Supplemental Table 6 Diagnostic parameters for the Healthy volunteers vs. Early CRC comparison (top) and associated ROC-curve (bottom) for GSH-S. Supplemental Table 7 Diagnostic parameters for the Healthy volunteers vs. Early CRC comparison (top) and associated ROC-curve (bottom) for cofilin-1 (CFL-1) (PDF 809 kb)
Rights and permissions
About this article
Cite this article
Strohkamp, S., Gemoll, T., Humborg, S. et al. Protein levels of clusterin and glutathione synthetase in platelets allow for early detection of colorectal cancer. Cell. Mol. Life Sci. 75, 323–334 (2018). https://doi.org/10.1007/s00018-017-2631-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2631-9