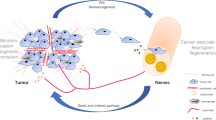
Abstract
Recent studies have demonstrated a critical role for nerves in enabling tumor progression. The association of nerves with cancer cells is well established for a variety of malignant tumors, including pancreatic, prostate and the head and neck cancers. This association is often correlated with poor prognosis. A strong partnership between cancer cells and nerve cells leads to both cancer progression and expansion of the nerve network. This relationship is supported by molecular pathways related to nerve growth and repair. Peripheral nerves form complex tumor microenvironments, which are made of several cell types including Schwann cells. Recent studies have revealed that Schwann cells enable cancer progression by adopting a de-differentiated phenotype, similar to the Schwann cell response to nerve trauma. A detailed understanding of the molecular and cellular mechanisms involved in the regulation of cancer progression by the nerves is essential to design strategies to inhibit tumor progression.


Similar content being viewed by others
References
Magnon C, Hall SJ, Lin J et al (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341:1236361. doi:10.1126/science.1236361
Zhao C-M, Hayakawa Y, Kodama Y et al (2014) Denervation suppresses gastric tumorigenesis. Sci Transl Med 6:250ra115–250ra115. doi:10.1126/scitranslmed.3009569
Peterson SC, Eberl M, Vagnozzi AN et al (2015) Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16:400–412. doi:10.1016/j.stem.2015.02.006
Saloman JL, Albers KM, Li D et al (2016) Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci. doi:10.1073/pnas.1512603113
Bockman DE, Büchler M, Beger HG (1994) Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 107:219–230
Amit M, Na’ara S, Gil Z (2016) Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. doi:10.1038/nrc.2016.38
Bapat AA, Hostetter G, Von Hoff DD, Han H (2011) Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 11:695–707. doi:10.1038/nrc3131
Demir IE, Ceyhan GO, Liebl F et al (2010) Neural invasion in pancreatic cancer: the past, present and future. Cancers 2:1513–1527. doi:10.3390/cancers2031513
Demir IE, Friess H, Ceyhan GO (2012) Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol 3:97. doi:10.3389/fphys.2012.00097
Liebig C, Ayala G, Wilks JA et al (2009) Perineural invasion in cancer. Cancer 115:3379–3391. doi:10.1002/cncr.24396
Batsakis JG (1985) Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 94:426–427
Petrou A, Soonawalla Z, Silva M-A et al (2016) Prognostic indicators following curative pancreatoduodenectomy for pancreatic carcinoma: a retrospective multivariate analysis of a single centre experience. J BUON 21:874–882
Nakao A, Harada A, Nonami T et al (1996) Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas 12:357–361
Mitsunaga S, Hasebe T, Kinoshita T et al (2007) Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol 31:1636–1644. doi:10.1097/PAS.0b013e318065bfe6
Eibl G, Reber HA (2005) A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas 31:258–262
Pour PM, Egami H, Takiyama Y (1991) Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology 100:529–536
Stopczynski RE, Normolle DP, Hartman DJ et al (2014) Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Can Res 74:1718–1727. doi:10.1158/0008-5472.CAN-13-2050
Amit M, Binenbaum Y, Trejo-Leider L et al (2015) International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 37:1038–1045. doi:10.1002/hed.23710
Chatterjee D, Katz MH, Rashid A et al (2012) Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol 36:409–417. doi:10.1097/PAS.0b013e31824104c5
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol (Lond). doi:10.1113/JP270874
Zochodne DW (2008) Neurobiology of peripheral nerve regeneration
Bunge MB, Wood PM, Tynan LB et al (1989) Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science 243:229–231
Kucenas S, Takada N, Park H-C et al (2008) CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci 11:143–151. doi:10.1038/nn2025
Cavel O, Shomron O, Shabtay A et al (2012) Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Can Res 72:5733–5743. doi:10.1158/0008-5472.CAN-12-0764
Deborde S, Omelchenko T, Lyubchik A et al (2016) Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126:1538–1554. doi:10.1172/JCI82658
Demir IE, Boldis A, Pfitzinger PL et al (2014) Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J Natl Cancer Inst 106:dju184. doi:10.1093/jnci/dju184
Sunami E, Kanazawa H, Hashizume H et al (2001) Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch Histol Cytol 64:191–201
Ushiki T, Ide C (1988) Autonomic nerve networks in the rat exocrine pancreas as revealed by scanning and transmission electron microscopy. Arch Histol Cytol 51:71–81
Ceyhan GO, Bergmann F, Kadihasanoglu M et al (2009) Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology 136(177–186):e1. doi:10.1053/j.gastro.2008.09.029
Pundavela J, Roselli S, Faulkner S et al (2015) Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol 9:1626–1635. doi:10.1016/j.molonc.2015.05.001
Jimenez-Andrade JM, Bloom AP, Stake JI et al (2010) Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci 30:14649–14656. doi:10.1523/JNEUROSCI.3300-10.2010
Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G et al (2011) Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 152:2564–2574. doi:10.1016/j.pain.2011.07.020
Mantyh WG, Jimenez-Andrade JM, Stake JI et al (2010) Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 171:588–598. doi:10.1016/j.neuroscience.2010.08.056
Ayala GE, Wheeler TM, Shine HD et al (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49:213–223. doi:10.1002/pros.1137
Polli-Lopes AC, Zucoloto S, de Queirós Cunha F et al (2003) Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett 190:45–50
Venkatesh HS, Johung TB, Caretti V et al (2015) Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161:803–816. doi:10.1016/j.cell.2015.04.012
Yamazaki S, Nakauchi H (2014) Bone marrow Schwann cells induce hematopoietic stem cell hibernation. Int J Hematol 99:695–698. doi:10.1007/s12185-014-1588-9
Pawlowski A, Weddell G (1967) Induction of tumours in denervated skin. Nature 213:1234–1236
Dubeykovskaya Z, Si Y, Chen X et al (2016) Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun 7:10517. doi:10.1038/ncomms10517
Kaminishi M, Shimizu N, Shimoyama S et al (1997) Denervation promotes the development of cancer-related lesions in the gastric remnant. J Clin Gastroenterol 25(Suppl 1):S129–S134
Lundegårdh G, Ekbom A, McLaughlin JK, Nyrén O (1994) Gastric cancer risk after vagotomy. Gut 35:946–949
Colomar A, Robitaille R (2004) Glial modulation of synaptic transmission at the neuromuscular junction. Glia 47:284–289. doi:10.1002/glia.20086
Wekerle H, Schwab M, Linington C, Meyermann R (1986) Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol 16:1551–1557. doi:10.1002/eji.1830161214
Shan C, Wei J, Hou R et al (2015) Schwann cells promote EMT and the Schwann-like differentiation of salivary adenoid cystic carcinoma cells via the BDNF/TrkB axis. Oncol Rep 35:427–435. doi:10.3892/or.2015.4366
Sroka IC, Chopra H, Das L et al (2015) Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem. doi:10.1002/jcb.25300
Ferguson TA, Muir D (2000) MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci 16:157–167. doi:10.1006/mcne.2000.0859
Abercrombie M (1979) Contact inhibition and malignancy. Nature 281:259–262. doi:10.1038/281259a0
Cravioto H (1965) The role of Schwann cells in the development of human peripheral nerves. An electron microscopic study. J Ultrastruct Res 12:634–651
Ben Q-W, Wang J-C, Liu J et al (2010) Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol 17:2213–2221. doi:10.1245/s10434-010-0955-x
Deborde S, Perret E, Gravotta D et al (2008) Clathrin is a key regulator of basolateral polarity. Nature 452:719–723. doi:10.1038/nature06828
Demir IE, Tieftrunk E, Schorn S et al (2016) Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. doi:10.1136/gutjnl-2015-309784
Jessen KR, Mirsky R, Lloyd AC (2015) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7:a020487. doi:10.1101/cshperspect.a020487
Fricker FR, Bennett DL (2011) The role of neuregulin-1 in the response to nerve injury. Future Neurol 6:809–822
Guertin AD, Zhang DP, Mak KS et al (2005) Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci 25:3478–3487. doi:10.1523/JNEUROSCI.3766-04.2005
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434. doi:10.1002/glia.20207
Imoto A, Mitsunaga S, Inagaki M et al (2012) Neural invasion induces cachexia via astrocytic activation of neural route in pancreatic cancer. Int J Cancer 131:2795–2807. doi:10.1002/ijc.27594
Clark CE, Hingorani SR, Mick R et al (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Can Res 67:9518–9527. doi:10.1158/0008-5472.CAN-07-0175
Demir IE, Schorn S, Schremmer-Danninger E et al (2013) Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One 8:e60529. doi:10.1371/journal.pone.0060529
Lesina M, Kurkowski MU, Ludes K et al (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19:456–469. doi:10.1016/j.ccr.2011.03.009
McAllister F, Bailey JM, Alsina J et al (2014) Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25:621–637. doi:10.1016/j.ccr.2014.03.014
Cattin A-L, Burden JJ, Van Emmenis L et al (2015) Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. doi:10.1016/j.cell.2015.07.021
Gaggioli C, Hooper S, Hidalgo-Carcedo C et al (2007) Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Publ Group 9:1392–1400. doi:10.1038/ncb1658
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563. doi:10.1038/nature06188
Vong S, Kalluri R (2011) The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer 2:1139–1145. doi:10.1177/1947601911423940
Merika EE, Syrigos KN, Saif MW (2012) Desmoplasia in pancreatic cancer. Can we fight it? Gastroenterol Res Pract 2012:781765–781810. doi:10.1155/2012/781765
Li X, Wang Z, Ma Q et al (2014) Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res 20:4326–4338. doi:10.1158/1078-0432.CCR-13-3426
Parrinello S, Napoli I, Ribeiro S et al (2010) EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143:145–155. doi:10.1016/j.cell.2010.08.039
Rhim AD, Oberstein PE, Thomas DH et al (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735–747. doi:10.1016/j.ccr.2014.04.021
Özdemir BC, Pentcheva-Hoang T, Carstens JL et al (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25:719–734. doi:10.1016/j.ccr.2014.04.005
Fields RD, Stevens-Graham B (2002) New insights into neuron-glia communication. Science 298:556–562. doi:10.1126/science.298.5593.556
Bakst RL, Wong RJ (2016) Mechanisms of perineural invasion. J Neurol Surg B Skull Base 77:96–106. doi:10.1055/s-0036-1571835
Ceyhan GO, Schäfer K-H, Kerscher AG et al (2010) Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg 251:923–931. doi:10.1097/SLA.0b013e3181d974d4
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. doi:10.1126/scisignal.2004088
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. doi:10.1158/2159-8290.CD-12-0095
Witkiewicz AK, McMillan EA, Balaji U et al (2015) Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 6:6744. doi:10.1038/ncomms7744
Beltran H, Prandi D, Mosquera JM et al (2016) Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22:298–305. doi:10.1038/nm.4045
Eirew P, Steif A, Khattra J et al (2015) Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518:422–426. doi:10.1038/nature13952
Ciriello G, Gatza ML, Beck AH et al (2015) Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163:506–519. doi:10.1016/j.cell.2015.09.033
Demir IE, Tieftrunk E, Schorn S et al (2016) Nerve growth factor and TrkA as novel therapeutic targets in cancer. Biochim Biophys Acta 1866:37–50. doi:10.1016/j.bbcan.2016.05.003
Zhu Z, Friess H, diMola FF et al (1999) Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol 17:2419–2428
Dang C, Zhang Y, Ma Q, Shimahara Y (2006) Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol 21:850–858. doi:10.1111/j.1440-1746.2006.04074.x
Ma J, Jiang Y, Jiang Y et al (2008) Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol 23:1852–1859. doi:10.1111/j.1440-1746.2008.05579.x
Sakamoto Y, Kitajima Y, Edakuni G et al (2001) Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol Rep 8:477–484
Chen-Tsai CP, Colome-Grimmer M, Wagner RF (2004) Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75NGFR, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg 30:1009–1016. doi:10.1111/j.1524-4725.2004.30306.x
Kolokythas A, Cox DP, Dekker N, Schmidt BL (2010) Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J Oral Maxillofac Surg 68:1290–1295. doi:10.1016/j.joms.2010.01.006
Kobayashi K, Ando M, Saito Y et al (2015) Nerve growth factor signals as possible pathogenic biomarkers for perineural invasion in adenoid cystic carcinoma. Otolaryngol Head Neck Surg 153:218–224. doi:10.1177/0194599815584762
Ketterer K, Rao S, Friess H et al (2003) Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res 9:5127–5136
Lewis Kelso R, Colome-Grimmer MI, Uchida T et al (2006) p75(NGFR) immunostaining for the detection of perineural invasion by cutaneous squamous cell carcinoma. Dermatol Surg 32:177–183
Verbeke S, Meignan S, Lagadec C et al (2010) Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1). Cell Signal 22:1864–1873. doi:10.1016/j.cellsig.2010.07.014
Johnston ALM, Lun X, Rahn JJ et al (2007) The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol 5:e212. doi:10.1371/journal.pbio.0050212
Jin H, Pan Y, Zhao L et al (2007) p75 neurotrophin receptor suppresses the proliferation of human gastric cancer cells. Neoplasia 9:471–478
Khwaja F, Tabassum A, Allen J, Djakiew D (2006) The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun 341:1184–1192. doi:10.1016/j.bbrc.2006.01.073
Tabassum A, Khwaja F, Djakiew D (2003) The p75(NTR) tumor suppressor induces caspase-mediated apoptosis in bladder tumor cells. Int J Cancer 105:47–52. doi:10.1002/ijc.11038
Taniuchi M, Clark HB, Schweitzer JB, Johnson EM (1988) Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci 8:664–681
Bentley CA, Lee KF (2000) p75 is important for axon growth and Schwann cell migration during development. J Neurosci 20:7706–7715
Sclabas GM, Fujioka S, Schmidt C et al (2005) Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res 11:440–449
Jia S, Wang W, Hu Z et al (2015) BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol 51:64–70. doi:10.1016/j.oraloncology.2014.10.008
Kowalski PJ, Paulino AFG (2002) Perineural invasion in adenoid cystic carcinoma: its causation/promotion by brain-derived neurotrophic factor. Hum Pathol 33:933–936. doi:10.1053/hupa.2002.128249
Johnson MD, Stone B, Thibodeau BJ et al (2017) The significance of Trk receptors in pancreatic cancer. Tumour Biol 39:1010428317692256. doi:10.1177/1010428317692256
Chopin V, Lagadec C, Toillon R-A, Le Bourhis X (2016) Neurotrophin signaling in cancer stem cells. Cell Mol Life Sci 73:1859–1870. doi:10.1007/s00018-016-2156-7
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Publ Group 8:755–768. doi:10.1038/nrc2499
Murillo-Sauca O, Chung MK, Shin JH et al (2014) CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget 5:6854–6866. doi:10.18632/oncotarget.2269
Redmer T, Welte Y, Behrens D et al (2014) The nerve growth factor receptor CD271 is crucial to maintain tumorigenicity and stem-like properties of melanoma cells. PLoS One 9:e92596. doi:10.1371/journal.pone.0092596
Tomellini E, Touil Y, Lagadec C et al (2015) Nerve growth factor and proNGF simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells 33:342–353. doi:10.1002/stem.1849
Yin B, Ma ZY, Zhou ZW et al (2015) The TrkB+ cancer stem cells contribute to post-chemotherapy recurrence of triple-negative breast cancers in an orthotopic mouse model. Oncogene 34:761–770. doi:10.1038/onc.2014.8
Gil Z, Cavel O, Kelly K et al (2010) Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst 102:107–118. doi:10.1093/jnci/djp456
He S, Chen C-H, Chernichenko N et al (2014) GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci. doi:10.1073/pnas.1402944111
Iwahashi N, Nagasaka T, Tezel G et al (2002) Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer 94:167–174
Liu H, Li X, Xu Q et al (2012) Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta 1826:112–120. doi:10.1016/j.bbcan.2012.03.010
Paratcha G, Ledda F, Ibáñez CF (2003) The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867–879
Naveilhan P, ElShamy WM, Ernfors P (1997) Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci 9:1450–1460
Gao L, Bo H, Wang Y et al (2015) Neurotrophic factor artemin promotes invasiveness and neurotrophic function of pancreatic adenocarcinoma in vivo and in vitro. Pancreas 44:134–143. doi:10.1097/MPA.0000000000000223
Wang K, Demir IE, D’Haese JG et al (2014) The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 35:103–113. doi:10.1093/carcin/bgt312
Ceyhan GO, Bergmann F, Kadihasanoglu M et al (2007) The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut 56:534–544. doi:10.1136/gut.2006.105528
Marchesi F, Locatelli M, Solinas G et al (2010) Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol 224:39–44. doi:10.1016/j.jneuroim.2010.05.007
Mehlen P, Delloye-Bourgeois C, Chédotal A (2011) Novel roles for slits and netrins: axon guidance cues as anticancer targets? Nat Publ Group 11:188–197. doi:10.1038/nrc3005
Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ (2016) Targeting the CCL2–CCR2 signaling axis in cancer metastasis. Oncotarget 7:28697–28710. doi:10.18632/oncotarget.7376
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274:1123–1133
Kameda K, Shimada H, Ishikawa T et al (1999) Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett 137:201–207
McLaughlin RB, Montone KT, Wall SJ et al (1999) Nerve cell adhesion molecule expression in squamous cell carcinoma of the head and neck: a predictor of propensity toward perineural spread. Laryngoscope 109:821–826
Seki H, Tanaka J, Sato Y et al (1993) Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol 53:78–83
Shang J, Sheng L, Wang K et al (2007) Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol Rep 18:1413–1416
Vural E, Hutcheson J, Korourian S et al (2000) Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg 122:717–720. doi:10.1067/mhn.2000.105057
Li R, Wheeler T, Dai H, Ayala G (2003) Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum Pathol. doi:10.1016/S0046-8177(03)00084-4
Binmadi NO, Yang YH, Zhou H et al (2012) Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. AJPA 180:1232–1242. doi:10.1016/j.ajpath.2011.12.009
Ding Y, He D, Florentin D et al (2013) Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res 19:6101–6111. doi:10.1158/1078-0432.CCR-12-3669
Dickson BJ, Gilestro GF (2006) Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22:651–675. doi:10.1146/annurev.cellbio.21.090704.151234
Ma L, Tessier-Lavigne M (2007) Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci 27:6843–6851. doi:10.1523/JNEUROSCI.1479-07.2007
Wu W, Wong K, Chen J et al (1999) Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 400:331–336. doi:10.1038/22477
Kinrade EF, Brates T, Tear G, Hidalgo A (2001) Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development 128:207–216
Wang Y, Teng H-L, Huang Z-H (2013) Repulsive migration of Schwann cells induced by Slit-2 through Ca2+-dependent RhoA-myosin signaling. Glia 61:710–723. doi:10.1002/glia.22464
Yiin J-J, Hu B, Jarzynka MJ et al (2009) Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol 11:779–789. doi:10.1215/15228517-2008-017
Göhrig A, Detjen KM, Hilfenhaus G et al (2014) Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Can Res 74:1529–1540. doi:10.1158/0008-5472.CAN-13-1012
Secq V, Leca J, Bressy C et al (2015) Stromal SLIT2 impacts on pancreatic cancer-associated neural remodeling. Cell Death Dis 6:e1592. doi:10.1038/cddis.2014.557
Shao Z, Zhu F, Song K et al (2013) EphA2/ephrinA1 mRNA expression and protein production in adenoid cystic carcinoma of salivary gland. J Oral Maxillofac Surg 71:869–878. doi:10.1016/j.joms.2012.10.026
Kinch MS, Moore M-B, Harpole DH (2003) Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res 9:613–618
Miyazaki T, Kato H, Fukuchi M et al (2003) EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer 103:657–663. doi:10.1002/ijc.10860
Afshari FT, Kwok JC, Fawcett JW (2010) Astrocyte-produced ephrins inhibit Schwann cell migration via VAV2 signaling. J Neurosci 30:4246–4255. doi:10.1523/JNEUROSCI.3351-09.2010
He S, He S, Chen C-H et al (2015) The chemokine (CCL2–CCR2) signaling axis mediates perineural invasion. Mol Cancer Res 13:380–390. doi:10.1158/1541-7786.MCR-14-0303
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266. doi:10.1016/j.cell.2006.01.007
Qian B-Z, Li J, Zhang H et al (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225. doi:10.1038/nature10138
Marchesi F, Piemonti L, Fedele G et al (2008) The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Can Res 68:9060–9069. doi:10.1158/0008-5472.CAN-08-1810
Lv C-Y, Zhou T, Chen W et al (2014) Preliminary study correlating CX3CL1/CX3CR1 expression with gastric carcinoma and gastric carcinoma perineural invasion. World J Gastroenterol 20:4428–4432. doi:10.3748/wjg.v20.i15.4428
Doumas S, Paterson JC, Norris PM et al (2015) Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) in squamous cell carcinoma of the tongue: markers of nerve invasion? Oral Maxillofac Surg 19:61–64. doi:10.1007/s10006-014-0455-4
Swanson BJ, McDermott KM, Singh PK et al (2007) MUC1 Is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Can Res 67:10222–10229. doi:10.1158/0008-5472.CAN-06-2483
Mukhopadhyay G, Doherty P, Walsh FS et al (1994) A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13:757–767
Nakamori S, Ota DM, Cleary KR et al (1994) MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 106:353–361
Lüttges J, Feyerabend B, Buchelt T et al (2002) The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 26:466–471
Kashiwagi H, Kijima H, Dowaki S et al (2000) DF3 expression in human gallbladder carcinoma: significance for lymphatic invasion. Int J Oncol 16:455–459
Nath S, Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20:332–342. doi:10.1016/j.molmed.2014.02.007
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deborde, S., Wong, R.J. How Schwann cells facilitate cancer progression in nerves. Cell. Mol. Life Sci. 74, 4405–4420 (2017). https://doi.org/10.1007/s00018-017-2578-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2578-x