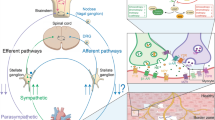
Abstract
In addition to traditional neurotransmitters of the sympathetic and parasympathetic nervous systems, the heart also contains numerous neuropeptides. These neuropeptides not only modulate the effects of neurotransmitters, but also have independent effects on cardiac function. While in most cases the physiological actions of these neuropeptides are well defined, their contributions to cardiac pathology are less appreciated. Some neuropeptides are cardioprotective, some promote adverse cardiac remodeling and heart failure, and in the case of others their functions are unclear. Some have both cardioprotective and adverse effects depending on the specific cardiac pathology and progression of that pathology. In this review, we briefly describe the actions of several neuropeptides on normal cardiac physiology, before describing in more detail their role in adverse cardiac remodeling and heart failure. It is our goal to bring more focus toward understanding the contribution of neuropeptides to the pathogenesis of heart failure, and to consider them as potential therapeutic targets.

Similar content being viewed by others
References
Habecker BA, Anderson ME, Birren SJ, Fukuda K, Herring N, Hoover DB, Kanazawa H, Paterson DJ, Ripplinger CM (2016) Molecular and cellular neurocardiology: Development, and cellular and molecular adaptations to heart disease. J Physiol 594:3853–3875
Gibbins IL, Furness JB, Costa M, Macintyre I, Hillyard CJ, Girgis S (1985) Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci Lett 57:125–130
Alevizaki M, Shiraishi A, Rassool FV, Ferrier GJ, Macintyre I, Legon S (1986) The calcitonin-like sequence of the beta CGRP gene. FEBS Lett 206:47–52
Steenbergh PH, Hoppener JW, Zandberg J, Visser A, Lips CJ, Jansz HS (1986) Structure and expression of the human calcitonin/CGRP genes. FEBS Lett 209:97–103
Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG (1985) Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 229:1094–1097
Morris HR, Panico M, Etienne T, Tippins J, Girgis SI, Macintyre I (1984) Isolation and characterization of human calcitonin gene-related peptide. Nature 308:746–748
Brain SD, Grant AD (2004) Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84:903–934
Mulderry PK, Ghatei MA, Bishop AE, Allen YS, Polak JM, Bloom SR (1985) Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul Pept 12:133–143
Fluhmann B, Muff R, Hunziker W, Fischer JA, Born W (1995) A human orphan calcitonin receptor-like structure. Biochem Biophys Res Commun 206:341–347
Mclatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM (1998) RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339
Muff R, Leuthauser K, Buhlmann N, Foord SM, Fischer JA, Born W (1998) Receptor activity modifying proteins regulate the activity of a calcitonin gene-related peptide receptor in rabbit aortic endothelial cells. FEBS Lett 441:366–368
Choksi T, Hay DL, Legon S, Poyner DR, Hagner S, Bloom SR, Smith DM (2002) Comparison of the expression of calcitonin receptor-like receptor (crlr) and receptor activity modifying proteins (RAMPS) with cgrp and adrenomedullin binding in cell lines. Br J Pharmacol 136:784–792
Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM (2000) CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275:31438–31443
Russell FA, King R, Smillei SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol Rev 94:1099–1142
Ando K, Pegram BL, Frohlich ED (1990) Hemodynamic effects of calcitonin gene-related peptide in spontaneously hypertensive rats. Am J Physiol 258:R425–R429
Gennari C, Nami R, Agnusdei D, Fischer JA (1990) Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc Res 24:239–241
Stevenson RN, Roberts RH, Timmis AD (1992) Calcitonin gene-related peptide: a haemodynamic study of a novel vasodilator in patients with severe chronic heart failure. Int J Cardiol 37:407–414
Li J, Levick SP, Dipette DJ, Janicki JS, Supowit SC (2013) Alpha-calcitonin gene-related peptide is protective against pressure overload-induced heart failure. Regul Pept 185C:20–28
Dubois-Rande JL, Merlet P, Benvenuti C, Sediame S, Macquin-Mavier I, Chabrier E, Braquet P, Castaigne A, Adnot S (1992) Effects of calcitonin gene-related peptide on cardiac contractility, coronary hemodynamics and myocardial energetics in idiopathic dilated cardiomyopathy. Am J Cardiol 70:906–912
Li J, Peng J, Wang C, Deng H, Li Y (2011) Calcitonin gene-related peptide suppresses isoprenaline-induced cardiomyocyte apoptosis through regulation of microrna-1 and microrna-133a expression. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:964–971
Mair J, Lechleitner P, Langle T, Wiedermann C, Dienstl F, Saria A (1990) Plasma CGRP in acute myocardial infarction. Lancet 335:168
Roudenok V, Gutjar L, Antipova V, Rogov Y (2001) Expression of vasoactive intestinal polypeptide and calcitonin gene-related peptide in human stellate ganglia after acute myocardial infarction. Ann Anat 183:341–344
Huang R, Karve A, Shah I, Bowers MC, Dipette DJ, Supowit SC, Abela GS (2008) Deletion of the mouse alpha-calcitonin gene-related peptide gene increases the vulnerability of the heart to ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 294:H1291–H1297
Li JZ, Peng J, Xiao L, Zhang YS, Liao MC, Li XH, Hu CP, Deng HW, Li YJ (2010) Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can. J Physiol Pharmacol 88:949–959
Von Euler US, Gaddum JH (1931) An unidentified depressor substance in certain tissue extracts. J Physiol 72:74–87
Steinhoff MS, Von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW (2014) Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol Rev 94:265–301
Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA (2004) Tachykinins and tachykinin receptors: a growing family. Life Sci 74:1445–1463
Page NM (2004) Hemokinins and endokinins. Cell Mol Life Sci 61:1652–1663
Dalsgaard CJ, Franco-Cereceda A, Saria A, Lundberg JM, Theodorsson-Norheim E, Hokfelt T (1986) Distribution and origin of substance P- and neuropeptide Y-immunoreactive nerves in the guinea-pig heart. Cell Tissue Res 243:477–485
Hougland MW, Hoover DB (1983) Detection of substance P-like immunoreactivity in nerve fibers in the heart of guinea-pigs but not rats. J Auton Nerv Syst 8:295–301
Papka RE, Urban L (1987) Distribution, origin and sensitivity to capsaicin of primary afferent substance P-immunoreactive nerves in the heart. Acta Physiol Hung 69:459–468
Reinecke M, Weihe E, Forssmann WG (1980) Substance P-immunoreactive nerve fibers in the heart. Neurosci Lett 20:265–269
Wharton J, Polak JM, Mcgregor GP, Bishop AE, Bloom SR (1981) The distribution of substrate P-like immunoreactive nerves in the guinea-pig heart. Neuroscience 6:2193–2204
Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, Pauza DH (2011) Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm 8:731–738
Milner P, Ralevic V, Hopwood AM, Feher E, Lincoln J, Kirkpatrick KA, Burnstock G (1989) Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia 45:121–125
Gerard NP, Bao L, Xiao-Ping H, Gerard C (1993) Molecular aspects of the tachykinin receptors. Regul Pept 43:21–35
Gerard NP, Garraway LA, Eddy RL Jr, Shows TB, Iijima H, Paquet JL, Gerard C (1991) Human substance P receptor (NK-1): Organization of the gene, chromosome localization, and functional expression of cDNA clones. BioChemistry 30:10640–10646
Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD (2009) Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol 30:271–276
Li H, Leeman SE, Slack BE, Hauser G, Saltsman WS, Krause JE, Blusztajn JK, Boyd ND (1997) A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci USA 94:9475–9480
Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M, Carletti R (2003) Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci 17:1736–1746
Mistrova E, Kruzliak P, Chottova DM (2016) Role of substance P in the cardiovascular system. Neuropeptides 58:41–51
Ustinova EE, Bergren D, Schultz HD (1995) Neuropeptide depletion impairs postischemic recovery of the isolated rat heart: Role of substance P. Cardiovasc Res 30:55–63
Wang L, Wang DH (2005) TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 112:3617–3623
Zhong B, Wang DH (2007) TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293:H1791–H1798
Ren JY, Song JX, Lu MY, Chen H (2011) Cardioprotection by ischemic postconditioning is lost in isolated perfused heart from diabetic rats: Involvement of transient receptor potential vanilloid 1, calcitonin gene-related peptide and substance P. Regul Pept 169:49–57
Jubair S, Li J, Dehlin HM, Manteufel EJ, Goldspink PH, Levick SP, Janicki JS (2015) Substance P induces cardioprotection in ischemia–reperfusion via activation of AKT. Am J Physiol Heart Circ Physiol 309:H676–H684
Ng LL, Sandhu JK, Narayan H et al (2014) Pro-substance P for evaluation of risk in acute myocardial infarction. J Am Coll Cardiol 64:1698–1707
Ejaz A, Logerfo FW, Khabbaz K, Pradhan L (2011) Expression of neuropeptide Y, substance P, and their receptors in the right atrium of diabetic patients. Clin Transl Sci 4:346–350
Yu Y, Liu L, Jiang JY, Qu XF, Yu G (2012) Parasympathetic and substance P-immunoreactive nerve denervation in atrial fibrillation models. Cardiovasc Pathol 21:39–45
Guler N, Ozkara C, Dulger H, Kutay V, Sahin M, Erbilen E, Gumrukcuoglu HA (2007) Do cardiac neuropeptides play a role in the occurrence of atrial fibrillation after coronary bypass surgery? Ann Thorac Surg 83:532–537
Mak IT, Chmielinska JJ, Kramer JH, Spurney CF, Weglicki WB (2011) Loss of neutral endopeptidase activity contributes to neutrophil activation and cardiac dysfunction during chronic hypomagnesemia: protection by substance P receptor blockade. Exp. Clin Cardiol 16:121–124
Weglicki WB, Phillips TM (1992) Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am. J Physiol 263:R734–R737
Weglicki WB, Mak IT, Phillips TM (1994) Blockade of cardiac inflammation in Mg2 + deficiency by substance P receptor inhibition. Circ Res 74:1009–1013
D’souza M, Garza MA, Xie M, Weinstock J, Xiang Q, Robinson P (2007) Substance P is associated with heart enlargement and apoptosis in murine dilated cardiomyopathy induced by taenia crassiceps infection. J Parasitol 93:1121–1127
Robinson P, Garza A, Moore J, Eckols TK, Parti S, Balaji V, Vallejo J, Tweardy DJ (2009) Substance P is required for the pathogenesis of EMCV infection in mice. Int J Clin Exp Med 2:76–86
Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP (2011) Substance P induces adverse myocardial remodeling via a mechanism involving cardiac mast cells. Cardiovasc Res 92:420–429
Dehlin HM, Manteufel EJ, Monroe AL, Reimer MH Jr, Levick SP (2013) Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int J Cardiol 168:4643–4651
Kumaran C, Shivakumar K (2002) Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. AJP—heart and circulatory. Physiology 282:H1855–H1862
Melendez GC, Manteufel EJ, Dehlin HM, Register TC, Levick SP (2015) Non-human primate and rat cardiac fibroblasts show similar extracellular matrix-related and cellular adhesion gene responses to substance P. Heart Lung Circ 24:395–403
Dehlin HM, Levick SP (2014) Substance P in heart failure: The good and the bad. Int J Cardiol 170:270–277
Church DJ, Arkinstall SJ, Vallotton MB, Chollet A, Kawashima E, Lang U (1996) Stimulation of atrial natriuretic peptide release by neurokinins in neonatal rat ventricular cardiomyocytes. Am J Physiol 270:H935–H944
Weglicki WB, Kramer JH, Spurney CF, Chmielinska JJ, Mak IT (2012) The EGFR tyrosine kinase inhibitor tyrphostin AG-1478 causes hypomagnesemia and cardiac dysfunction. Can J Physiol Pharmacol 90:1145–1149
Mak IT, Kramer JH, Chmielinska JJ, Spurney CF, Weglicki WB (2015) EGFR-TKI, erlotinib, causes hypomagnesemia, oxidative stress, and cardiac dysfunction: Attenuation by NK-1 receptor blockade. J Cardiovasc Pharmacol 65:54–61
Angsutararux P, Luanpitpong S, Issaragrisil S (2015) Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev 2015:795602
Robinson P, Kasembeli M, Bharadwaj U, Engineer N, Eckols KT, Tweardy DJ (2016) Substance P receptor signaling mediates doxorubicin-induced cardiomyocyte apoptosis and triple-negative breast cancer chemoresistance. Biomed. Res Int 2016:1959270
Munoz M, Covenas R (2013) Safety of neurokinin-1 receptor antagonists. Expert Opin Drug Saf 12:673–685
Campbell G, Gibbins IL, Morris JL, Furness JB, Costa M, Oliver JR, Beardsley AM, Murphy R (1982) Somatostatin is contained in and released from cholinergic nerves in the heart of the toad bufo marinus. Neuroscience 7:2013–2023
Day SM, Gu J, Polak JM, Bloom SR (1985) Somatostatin in the human heart and comparison with guinea pig and rat heart. Br Heart J 53:153–157
Smith WH, Nair RU, Adamson D, Kearney MT, Ball SG, Balmforth AJ (2005) Somatostatin receptor subtype expression in the human heart: differential expression by myocytes and fibroblasts. J Endocrinol 187:379–386
Ohmura T, Nishio M, Kigoshi S, Muramatsu I (1990) Somatostatin decreases the calcium inward current in guinea-pig atria. Br J Pharmacol 99:587–591
Lewis DL, Clapham DE (1989) Somatostatin activates an inwardly rectifying K+ channel in neonatal rat atrial cells. Pflugers Arch 414:492–494
Franco-Cereceda A, Lundberg JM, Hokfelt T (1986) Somatostatin: an inhibitory parasympathetic transmitter in the human heart? Eur J Pharmacol 132:101–102
Rettig R, Geist R, Sauer U, Rohmeiss P, Unger T (1989) Central effects of somatostatin: Pressor response, avp release, and sympathoinhibition. Am J Physiol 257:R588–R594
Brown MR (1988) Somatostatin-28 effects on central nervous system regulation of vasopressin secretion and blood pressure. Neuroendocrinology 47:556–562
Hirai S, Hasegawa J, Mashiba H (1989) Positive inotropic effect of somatostatin in guinea-pig ventricular muscles. J Mol Cell Cardiol 21:607–616
Erbas T, Usman A, Erbas B, Varoglu E, Aras T, Bekdik C (1993) Short-term effects of somatostatin analogue (SMS 201–995) on left ventricular function in healthy persons: a scintigraphic study. J Endocrinol Invest 16:857–861
Grant MB, Wargovich TJ, Ellis EA, Caballero S, Mansour M, Pepine CJ (1994) Localization of insulin-like growth factor I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. A potential treatment for restenosis? Circulation 89:1511–1517
Gunal AI, Isik A, Celiker H, Eren O, Celebi H, Gunal SY, Luleci C (1996) Short term reduction of left ventricular mass in primary hypertrophic cardiomyopathy by octreotide injections. Heart 76:418–421
Demirtas E, Sag C, Kursaklioglu H, Uzun M, Uzbay T, Tore HF, Kose S, Genc C, Demirkan D (1998) Effects of octreotide in patients with hypertrophic obstructive cardiomyopathy. Jpn Heart J 39:173–181
Leszczynski D, Josephs MD, Fournier RS, Foegh ML (1993) Angiopeptin, the octapeptide analogue of somatostatin, decreases rat heart endothelial cell adhesiveness for mononuclear cells. Regul Pept 43:131–140
Webb SC, Krikler DM, Hendry WG, Adrian TE, Bloom SR (1986) Electrophysiological actions of somatostatin on the atrioventricular junction in sinus rhythm and reentry tachycardia. Br Heart J 56:236–241
Merola B, Cittadini A, Colao A, Ferone D, Fazio S, Sabatini D, Biondi B, Sacca L, Lombardi G (1993) Chronic treatment with the somatostatin analog octreotide improves cardiac abnormalities in acromegaly. J Clin Endocrinol Metab 77:790–793
Bogazzi F, Lombardi M, Strata E et al (2010) Effects of somatostatin analogues on acromegalic cardiomyopathy: results from a prospective study using cardiac magnetic resonance. J Endocrinol Invest 33:103–108
Bogazzi F, Di Bello V, Palagi C et al (2005) Improvement of intrinsic myocardial contractility and cardiac fibrosis degree in acromegalic patients treated with somatostatin analogues: a prospective study. Clin Endocrinol 62:590–596
Comunello A, Dassie F, Martini C et al (2015) Heart rate variability is reduced in acromegaly patients and improved by treatment with somatostatin analogues. Pituitary 18:525–534
Russell FD, Meyers D, Galbraith AJ, Bett N, Toth I, Kearns P, Molenaar P (2003) Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am J Physiol Heart Circ Physiol 285:H1576–H1581
Bohm F, Pernow J (2002) Urotensin II evokes potent vasoconstriction in humans in vivo. Br J Pharmacol 135:25–27
Malagon MM, Molina M, Gahete MD et al (2008) Urotensin II and urotensin II-related peptide activate somatostatin receptor subtypes 2 and 5. Peptides 29:711–720
Ames RS, Sarau HM, Chambers JK et al (1999) Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 401:282–286
Lapp H, Boerrigter G, Costello-Boerrigter LC, Jaekel K, Scheffold T, Krakau I, Schramm M, Guelker H, Stasch JP (2004) Elevated plasma human urotensin-II-like immunoreactivity in ischemic cardiomyopathy. Int J Cardiol 94:93–97
Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, Kelly DJ, Gilbert RE, Krum H (2003) Direct actions of urotensin II on the heart: Implications for cardiac fibrosis and hypertrophy. Circ Res 93:246–253
Zhang Y, Li J, Cao J, Chen J, Yang J, Zhang Z, Du J, Tang C (2002) Effect of chronic hypoxia on contents of urotensin II and its functional receptors in rat myocardium. Heart Vessels 16:64–68
Dai HY, Guo XG, Ge ZM, Li ZH, Yu XJ, Tang MX, Zhang Y (2008) Elevated expression of urotensin II and its receptor in diabetic cardiomyopathy. J Diabetes Complications 22:137–143
Ross B, Mckendy K, Giaid A (2010) Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol 298:R1156–R1172
Henning RJ, Sawmiller DR (2001) Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49:27–37
Forssmann WG, Triepel J, Daffner C, Heym C, Cuevas P, Noble MI, Yanaihara N (1988) Vasoactive intestinal peptide in the heart. Ann N Y Acad Sci 527:405–420
Dvorakova MC, Pfeil U, Kuncova J et al (2006) Down-regulation of vasoactive intestinal peptide and altered expression of its receptors in rat diabetic cardiomyopathy. Cell Tissue Res 323:383–393
Rigel DF (1988) Effects of neuropeptides on heart rate in dogs: comparison of VIP, PHI, NPY, CGRP, and NT. Am J Physiol 255:H311–H317
Rigel DF, Grupp IL, Balasubramaniam A, Grupp G (1989) Contractile effects of cardiac neuropeptides in isolated canine atrial and ventricular muscles. Am J Physiol 257:H1082–H1087
Sawmiller DR, Ashtari M, Urueta H, Leschinsky M, Henning RJ (2006) Mechanisms of vasoactive intestinal peptide-elicited coronary vasodilation in the isolated perfused rat heart. Neuropeptides 40:349–355
Nicholls DP, Riley M, Elborn JS, Stanford CF, Shaw C, Mckillop JM, Buchanan KD (1992) Regulatory peptides in the plasma of patients with chronic cardiac failure at rest and during exercise. Eur Heart J 13:1399–1404
Kupari M, Mikkola TS, Turto H, Lommi J, Ylikorkala O (2006) Vasoactive intestinal peptide—release from the heart and response in heart failure due to left ventricular pressure overload. Eur J Heart Fail 8:361–365
Lucia P, Caiola S, Coppola A, Manetti LL, Maroccia E, Buongiorno AM, De Martinis C (2003) Vasoactive intestinal peptide (VIP): a new neuroendocrine marker of clinical progression in chronic heart failure? Clin Endocrinol (Oxf) 59:723–727
Alston EN, Parrish DC, Hasan W, Tharp K, Pahlmeyer L, Habecker BA (2011) Cardiac ischemia–reperfusion regulates sympathetic neuropeptide expression through gp130-dependent and independent mechanisms. Neuropeptides 45:33–42
Kalfin R, Maulik N, Engelman RM, Cordis GA, Milenov K, Kasakov L, Das DK (1994) Protective role of intracoronary vasoactive intestinal peptide in ischemic and reperfused myocardium. J Pharmacol Exp Ther 268:952–958
Szema AM, Hamidi SA, Smith SD, Benveniste H (2013) VIP gene deletion in mice causes cardiomyopathy associated with upregulation of heart failure genes. PLoS One 8:e61449.
Szema AM, Hamidi SA (2014) Gene deletion of VIP leads to increased mortality associated with progressive right ventricular hypertrophy. J Cardiovasc Dis 2:131–136
Szema AM, Dang S, Li JC (2015) Emerging novel therapies for heart failure. Clin Med Insights Cardiol 9:57–64
Brack KE, Coote JH, Ng GA (2011) Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res 91:437–446
Osadchii OE (2015) Emerging role of neurotensin in regulation of the cardiovascular system. Eur J Pharmacol 762:184–192
Ceconi C, Condorelli E, Quinzanini M, Rodella A, Ferrari R, Harris P (1989) Noradrenaline, atrial natriuretic peptide, bombesin and neurotensin in myocardium and blood of rats in congestive cardiac failure. Cardiovasc Res 23:674–682
Osadchii O, Norton G, Deftereos D, Badenhorst D, Woodiwiss A (2005) Impact and mechanisms of action of neurotensin on cardiac contractility in the rat left ventricle. Eur J Pharmacol 520:108–117
Reinecke M, Weihe E, Carraway RE, Leeman SE, Forssmann WG (1982) Localization of neurotensin immunoreactive nerve fibers in the guinea-pig heart: evidence derived by immunohistochemistry, radioimmunoassay and chromatography. Neuroscience 7:1785–1795
Florholmen G, Andersson KB, Yndestad A, Austbo B, Henriksen UL, Christensen G (2004) Leukaemia inhibitory factor alters expression of genes involved in rat cardiomyocyte energy metabolism. Acta Physiol Scand 180:133–142
Quirion R, Rioux F, Regoli D, St Pierre S (1980) Selective blockade of neurotensin-induced coronary vessel constriction in perfused rat hearts by a neurotensin analogue. Eur J Pharmacol 61:309–312
Quirion R, Rioux F, Regoli D, St Pierre S (1980) Pharmacological studies of neurotensin, several fragments and analogous in the isolated perfused rat heart. Eur J Pharmacol 66:257–266
Bachelard H, St Pierre S, Rioux F (1986) The coronary vasodilator effect of neurotensin in the guinea pig isolated heart. Peptides 7:431–435
Ertl G, Bauer B, Becker HH, Rose G (1993) Effects of neurotensin and neuropeptide Y on coronary circulation and myocardial function in dogs. Am J Physiol 264:H1062–H1068
Quirion R, Rioux F, Regoli D (1978) Chronotropic and inotropic effects of neurotensin on spontaneously beating auricles. Can. J Physiol Pharmacol 56:671–673
Bachelard H, St Pierre S, Rioux F (1985) The chronotropic action of neurotensin in the guinea pig isolated heart. Peptides 6:841–845
Osadchii O, Norton G, Deftereos D, Muller D, Woodiwiss A (2006) Impact of chronic beta-adrenoceptor activation on neurotensin-induced myocardial effects in rats. Eur J Pharmacol 553:246–253
Osadchii O, Woodiwiss A, Deftereos D, Norton G (2006) Neurotensin-induced myocardial noradrenergic effects in spontaneously hypertensive rats. J Cardiovasc Pharmacol 47:221–227
Rioux F, Kerouac R, St-Pierre S (1985) Characterization of the histamine releasing effect of neurotensin in the rat heart. Peptides 6:121–125
Levick SP, Mclarty JL, Murray DB, Freeman RM, Carver WE, Brower GL (2009) Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension 53:1041–1047
Levick SP, Melendez GC, Plante E, Mclarty JL, Brower GL, Janicki JS (2011) Cardiac mast cells: The centrepiece in adverse myocardial remodelling. Cardiovasc Res 89:12–19
Mclarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP (2011) Tryptase/protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension 58:264–270
Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS (2002) Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol 283:H518–H525
Brower GL, Janicki JS (2005) Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail 11:548–556
Stewart JA, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell’italia LJ (2003) Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 35:311–319
Patella V, De Crescenzo G, Lamparter-Schummert B, De Rosa G, Adt M, Marone G (1997) Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res 46:S31–S32
Balakumar P, Singh AP, Ganti SS, Krishan P, Ramasamy S, Singh M (2008) Resident cardiac mast cells: are they the major culprit in the pathogenesis of cardiac hypertrophy? Basic Clin. Pharmacol Toxicol 102:5–9
Batlle M, Roig E, Perez-Villa F et al (2006) Increased expression of the renin-angiotensin system and mast cell density but not of angiotensin-converting enzyme II in late stages of human heart failure. J Heart Lung Transplant 25:1117–1125
Scarpa RC, Carraway RE, Cochrane DE (2004) The effect of neurotensin on insulin-induced proliferation of human fibroblasts. Peptides 25:1159–1169
Melander O, Maisel AS, Almgren P et al (2012) Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 308:1469–1475
McDermott BJ, Bell D (2007) NPY and cardiac diseases. Curr Top Med Chem 7:1692–1703
McDermott BJ, Millar BC, Dolan FM, Bell D, Balasubramaniam A (1997) Evidence for Y1 and Y2 subtypes of neuropeptide Y receptors linked to opposing postjunctional effects observed in rat cardiac myocytes. Eur J Pharmacol 336:257–265
Callanan EY, Lee EW, Tilan JU, Winaver J, Haramati A, Mulroney SE, Zukowska Z (2007) Renal and cardiac neuropeptide Y and NPY receptors in a rat model of congestive heart failure. Am J Physiol Renal Physiol 293:F1811–F1817
Matyal R, Mahmood F, Robich M et al (2011) Chronic type II diabetes mellitus leads to changes in neuropeptide Y receptor expression and distribution in human myocardial tissue. Eur J Pharmacol 665:19–28
Raimondi L, Banchelli G, Matucci R, Stillitano F, Pirisino R (2002) The direct stimulation of Gi proteins by neuropeptide Y (NPY) in the rat left ventricle. Biochem Pharmacol 63:2063–2068
Onuoha GN, Nicholls DP, Alpar EK, Ritchie A, Shaw C, Buchanan K (1999) Regulatory peptides in the heart and major vessels of man and mammals. Neuropeptides 33:165–172
Haass M, Cheng B, Richardt G, Lang RE, Schomig A (1989) Characterization and presynaptic modulation of stimulation-evoked exocytotic co-release of noradrenaline and neuropeptide Y in guinea pig heart. Naunyn Schmiedebergs Arch Pharmacol 339:71–78
Heredia MP, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, Benitah JP, Gomez AM (2005) Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2 + sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol 38:205–212
Piper HM, Millar BC, McDermott BJ (1989) The negative inotropic effect of neuropeptide Y on the ventricular cardiomyocyte. Naunyn Schmiedebergs Arch Pharmacol 340:333–337
Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ (2008) Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J Mol Cell Cardiol 44:477–485
Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K (2015) Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 12:1027–1035
Ullman B, Franco-Cereceda A, Hulting J, Lundberg JM, Sollevi A (1990) Elevation of plasma neuropeptide Y-like immunoreactivity and noradrenaline during myocardial ischaemia in man. J Intern Med 228:583–589
Liu JJ, Shi SG, Han QD (1994) Evaluation of plasma neuropeptide Y levels in patients with congestive heart failure. Zhonghua Nei Ke Za Zhi 33:687–689
Ullman B, Hulting J, Lundberg JM (1994) Prognostic value of plasma neuropeptide-Y in coronary care unit patients with and without acute myocardial infarction. Eur Heart J 15:454–461
Cuculi F, Herring N, De Caterina AR, Banning AP, Prendergast BD, Forfar JC, Choudhury RP, Channon KM, Kharbanda RK (2013) Relationship of plasma neuropeptide Y with angiographic, electrocardiographic and coronary physiology indices of reperfusion during ST elevation myocardial infarction. Heart 99:1198–1203
Clarke JG, Davies GJ, Kerwin R et al (1987) Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet 1:1057–1059
Maisel AS, Scott NA, Motulsky HJ, Michel MC, Boublik JH, Rivier JE, Ziegler M, Allen RS, Brown MR (1989) Elevation of plasma neuropeptide Y levels in congestive heart failure. Am J Med 86:43–48
Szardien S, Mollmann H, Voss S et al (2011) Elevated serum levels of neuropeptide Y in stress cardiomyopathy. Int J Cardiol 147:155–157
Yalta K, Sivri N, Yalta T (2011) Neuropeptide Y-induced coronary microvascular dysfunction: a significant contributor to the adverse outcomes in stress cardiomyopathy? Int J Cardiol 147:284
Ilhan A, Rasul S, Dimitrov A, Handisurya A, Gartner W, Baumgartner-Parzer S, Wagner L, Kautzky-Willer A, Base W (2010) Plasma neuropeptide Y levels differ in distinct diabetic conditions. Neuropeptides 44:485–489
Costoli T, Sgoifo A, Stilli D, Flugge G, Adriani W, Laviola G, Fuchs E, Pedrazzini T, Musso E (2005) Behavioural, neural and cardiovascular adaptations in mice lacking the NPY Y1 receptor. Neurosci Biobehav Rev 29:113–123
Millar BC, Schluter KD, Zhou XJ, McDermott BJ, Piper HM (1994) Neuropeptide Y stimulates hypertrophy of adult ventricular cardiomyocytes. Am J Physiol 266:C1271–C1277
Bell D, Allen AR, Kelso EJ, Balasubramaniam A, McDermott BJ (2002) Induction of hypertrophic responsiveness of cardiomyocytes to neuropeptide Y in response to pressure overload. J Pharmacol Exp Ther 303:581–591
Omerovic E, Ramunddal T, Lorentzon M, Nordlander M (2007) Effects of neuropeptide Y2 receptor blockade on ventricular arrhythmias in rats with acute myocardial infarction. Eur J Pharmacol 565:138–143
Luo G, Xu X, Guo W, Luo C, Wang H, Meng X, Zhu S, Wei Y (2015) Neuropeptide Y damages the integrity of mitochondrial structure and disrupts energy metabolism in cultured neonatal rat cardiomyocytes. Peptides 71:162–169
Matyal R, Sakamuri S, Wang A, Mahmood E, Robich MP, Khabbaz K, Hess PE, Sellke FW, Mahmood F (2013) Local infiltration of neuropeptide Y as a potential therapeutic agent against apoptosis and fibrosis in a swine model of hypercholesterolemia and chronic myocardial ischemia. Eur J Pharmacol 718:261–270
Matyal R, Chu L, Mahmood F et al (2012) Neuropeptide Y improves myocardial perfusion and function in a swine model of hypercholesterolemia and chronic myocardial ischemia. J Mol Cell Cardiol 53:891–898
Chen X, Lu G, Tang K, Li Q, Gao X (2015) The secretion patterns and roles of cardiac and circulating arginine vasopressin during the development of heart failure. Neuropeptides 51:63–73
Hupf H, Grimm D, Riegger GA, Schunkert H (1999) Evidence for a vasopressin system in the rat heart. Circ Res 84:365–370
Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, Morgenthaler NG, Davies JE, Ng LL (2008) C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J Card Fail 14:739–745
Carmichael MC, Kumar R (1994) Molecular biology of vasopressin receptors. Semin Nephrol 14:341–348
Xu YJ, Gopalakrishnan V (1991) Vasopressin increases cytosolic free [Ca2+] in the neonatal rat cardiomyocyte. Evidence for V1 subtype receptors. Circ Res 69:239–245
Schweiger TA, Zdanowicz MM (2008) Vasopressin-receptor antagonists in heart failure. Am J Health Syst Pharm 65:807–817
Rehsia NS, Dhalla NS (2010) Potential of endothelin-1 and vasopressin antagonists for the treatment of congestive heart failure. Heart Fail Rev 15:85–101
Zhu W, Tilley DG, Myers VD, Tsai EJ, Feldman AM (2014) Increased vasopressin 1a receptor expression in failing human hearts. J Am Coll Cardiol 63:375–376
Tahara A, Tomura Y, Wada K, Kusayama T, Tsukada J, Ishii N, Yatsu T, Uchida W, Tanaka A (1998) Effect of YM087, a potent nonpeptide vasopressin antagonist, on vasopressin-induced protein synthesis in neonatal rat cardiomyocyte. Cardiovasc Res 38:198–205
Xie Z, Gao M, Batra S, Koyama T (1997) Remodeling of capillary network in left ventricular subendocardial tissues induced by intravenous vasopressin administration. Microcirculation 4:261–266
Rapoport B, Mclachlan SM (2016) TSH receptor cleavage into subunits and shedding of the A-subunit; a molecular and clinical perspective. Endocr Rev 37:114–134
Ga D (2016) Thyroid hormones and the heart. Heart Fail Rev 21:357–359
Wang XH, Wang WF, Cao YX, Ma AQ (2013) Ang II receptor expression and effect of ang II receptor blockade in thyrotoxic rat myocardium. Eur Rev Med Pharmacol Sci 17:2619–2627
Cokkinos DV, Chryssanthopoulos S (2016) Thyroid hormones and cardiac remodeling. Heart Fail Rev 21:365–372
Sugiura T, Yamanaka S, Takeuchi H, Morimoto N, Kamioka M, Matsumura Y (2015) Autoimmunity and pulmonary hypertension in patients with graves’ disease. Heart Vessels 30:642–646
Levick S, Fenning A, L B (2005) Increases calcium influx mediates increased cardiac stiffness in hyperthyroid rats. Cell Biochem Biophys 43:53–60
Klein I (1990) Thyroid hormone and the cardiovascular system. Am J Med 88:631–637
Liu L, Yun F, Zhao H et al (2015) Atrial sympathetic remodeling in experimental hyperthyroidism and hypothyroidism rats. Int J Cardiol 187:148–150
Klein I, Danzi S (2007) Thyroid disease and the heart. Circulation 116:1725–1735
Pol CJ, Muller A, Simonides WS (2010) Cardiomyocyte-specific inactivation of thyroid hormone in pathologic ventricular hypertrophy: an adaptative response or part of the problem? Heart Fail Rev 15:133–142
Galli E, Pingitore A, Iervasi G (2010) The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev 15:155–169
Mazza R, Tota B, Gattuso A (2015) Cardio-vascular activity of catestatin: Interlocking the puzzle pieces. Curr Med Chem 22:292–304
Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC (2008) The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793
Peng F, Chu S, Ding W, Liu L, Zhao J, Cui X, Li R, Wang J (2016) The predictive value of plasma catestatin for all-cause and cardiac deaths in chronic heart failure patients. Peptides 86:112–117
Xu W, Yu H, Wu H, Li S, Chen B, Gao W (2016) Plasma catestatin in patients with acute coronary syndrome. Cardiology 136:164–169
Wang D, Liu T, Shi S, Li R, Shan Y, Huang Y, Hu D, Huang C (2016) Chronic administration of catestatin improves autonomic function and exerts cardioprotective effects in myocardial infarction rats. J Cardiovasc Pharmacol Ther 21:526–535
Penna C, Pasqua T, Amelio D, et al. (2014) Catestatin increases the expression of anti-apoptotic and pro-angiogenetic factors in the post-ischemic hypertrophied heart of SHR. PLoS One 9:e102536.
Bassino E, Fornero S, Gallo MP, Gallina C, Femmino S, Levi R, Tota B, Alloatti G (2015) Catestatin exerts direct protective effects on rat cardiomyocytes undergoing ischemia/reperfusion by stimulating PI3K-AKT-GSK3beta pathway and preserving mitochondrial membrane potential. PLoS One 10:e0119790
Kirchmair R, Marksteiner J, Troger J et al (1994) Human and rat primary c-fibre afferents store and release secretoneurin, a novel neuropeptide. Eur J Neurosci 6:861–868
Chan CK, Vanhoutte PM (2011) Secretoneurin facilitates endothelium-dependent relaxations in porcine coronary arteries. Am J Physiol Heart Circ Physiol 300:H1159–H1165
Rosjo H, Stridsberg M, Florholmen G, et al. (2012) Secretogranin II; a protein increased in the myocardium and circulation in heart failure with cardioprotective properties. PLoS One 7:e37401
Ottesen AH, Louch WE, Carlson CR et al (2015) Secretoneurin is a novel prognostic cardiovascular biomarker associated with cardiomyocyte calcium handling. J Am Coll Cardiol 65:339–351
Albrecht-Schgoer K, Schgoer W, Holfeld J et al (2012) The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 126:2491–2501
Herring N, Cranley J, Lokale MN et al (2012) The cardiac sympathetic co-transmitter galanin reduces acetylcholine release and vagal bradycardia: implications for neural control of cardiac excitability. J Mol Cell Cardiol 52:667–676
Fang P, Sun J, Wang X, Zhang Z, Bo P, Shi M (2013) Galanin participates in the functional regulation of the diabetic heart. Life Sci 92:628–632
Kocic I (1998) The influence of the neuropeptide galanin on the contractility and the effective refractory period of guinea-pig heart papillary muscle under normoxic and hypoxic conditions. J Pharm Pharmacol 50:1361–1364
Habecker BA, Gritman KR, Willison BD, Van Winkle DM (2005) Myocardial infarction stimulates galanin expression in cardiac sympathetic neurons. Neuropeptides 39:89–95
Chen A, Li M, Song L, Zhang Y, Luo Z, Zhang W, Chen Y, He B (2015) Effects of the galanin receptor antagonist M40 on cardiac function and remodeling in rats with heart failure. Cardiovasc Ther 33:288–293
Fang P, Yu M, Gu X, Shi M, Zhu Y, Zhang Z, Bo P (2016) Low levels of plasma galanin in obese subjects with hypertension. J Endocrinol Invest
Fang P, Shi M, Guo L, He B, Wang Q, Yu M, Bo P, Zhang Z (2014) Effect of endogenous galanin on glucose transporter 4 expression in cardiac muscle of type 2 diabetic rats. Peptides 62:159–163
Fang P, Shi M, Zhu Y, Zhang Z, Bo P (2015) Central injection of Galr1 agonist M617 facilitates Glut4 expression in cardiac muscle of type 2 diabetic rats. Exp Gerontol 65:85–89
Brain SD, Cox HM (2006) Neuropeptides and their receptors: Innovative science providing novel therapeutic targets. Br. J Pharmacol 147(Suppl 1):S202–S211
Hoover DB, Chang Y, Hancock JC, Zhang L (2000) Actions of tachykinins within the heart and their relevance to cardiovascular disease. Jpn J Pharmacol 84:367–373
Hoover DB, Chang Y, Hancock JC (1998) Characterization of responses to neurokinin A in the isolated perfused guinea pig heart. Am J Physiol 275:R1803–R1811
Gulati N, Mathison R, Huggel H, Regoli D, Beny JL (1987) Effects of neurokinins on the isolated pig coronary artery. Eur J Pharmacol 137:149–154
Hoover DB, Hossler FE (1993) Vasoconstrictor and dilator responses to neurokinin A in isolated guinea pig heart. Peptides 14:29–36
Hernandez JM, Cox G, Janssen LJ (2008) Involvement of the neurokinin-2 receptor in airway smooth muscle stretch-activated contractions assessed in perfused intact bovine bronchial segments. J Pharmacol Exp Ther 327:503–510
Miotto D, Boschetto P, Cavallesco G, Zeni E, Querzoli P, Pedriali M, Chiarelli S, Fabbri LM, Mapp CE (2007) Increased neurokinin-2 receptor expression in alveolar macrophages of smokers with COPD. Histopathology 51:128–131
Sculptoreanu A, Aura KF, De Groat WC (2008) Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in drg neurons from adult rats. Eur J Neurosci 27:3171–3181
Michalski CW, Shi X, Reiser C, Fachinger P, Zimmermann A, Buchler MW, Di Sebastiano P, Friess H (2007) Neurokinin-2 receptor levels correlate with intensity, frequency, and duration of pain in chronic pancreatitis. Ann Surg 246:786–793
Kitamura H, Kobayashi M, Wakita D, Nishimura T (2012) Neuropeptide signaling activates dendritic cell-mediated type 1 immune responses through neurokinin-2 receptor. J Immunol 188:4200–4208
Hastrup H, Schwartz TW (1996) Septide and neurokinin A are high-affinity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS Lett 399:264–266
Maggi CA, Schwartz TW (1997) The dual nature of the tachykinin NK1 receptor. Trends Pharmacol Sci 18:351–355
Picard P, Regoli D, Couture R (1994) Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br J Pharmacol 112:240–249
Tschope C, Picard P, Culman J, Prat A, Itoi K, Regoli D, Unger T, Couture R (1992) Use of selective antagonists to dissociate the central cardiovascular and behavioural effects of tachykinins on NK1 and NK2 receptors in the rat. Br J Pharmacol 107:750–755
Wijkhuisen A, Sagot MA, Frobert Y, Creminon C, Grassi J, Boquet D, Couraud JY (1999) Identification in the NK1 tachykinin receptor of a domain involved in recognition of neurokinin A and septide but not of substance P. FEBS Lett 447:155–159
Tauer U, Zhao Y, Hunt SP, Culman J (2012) Are biological actions of neurokinin A in the adult brain mediated by a cross-talk between the NK1 and NK2 receptors? Neuropharmacology 63:958–965
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health HL-093215 (S.P.L.), HL-132908 (S.P.L.), and the Greater Milwaukee Foundation-Elsa Schoeneich Medical Research Fund (S.P.L.).
Rights and permissions
About this article
Cite this article
Widiapradja, A., Chunduri, P. & Levick, S.P. The role of neuropeptides in adverse myocardial remodeling and heart failure. Cell. Mol. Life Sci. 74, 2019–2038 (2017). https://doi.org/10.1007/s00018-017-2452-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2452-x