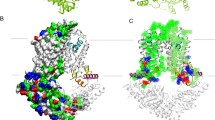
Abstract
The ATP-binding cassette (ABC) transporters of class G display a different domain organisation than P-glycoprotein/ABCB1 and bacterial homologues with a nucleotide-binding domain preceding the transmembrane domain. The linker region connecting these domains is unique and its function and structure cannot be predicted. Sequence analysis revealed that the human ABCG2 linker contains a LSGGE sequence, homologous to the canonical C-motif/ABC signature present in all ABC nucleotide-binding domains. Predictions of disorder and of secondary structures indicated that this C2-sequence was highly mobile and located between an α-helix and a loop similarly to the C-motif. Point mutations of the two first residues of the C2-sequence fully abolished the transport-coupled ATPase activity, and led to the complete loss of cell resistance to mitoxantrone. The interaction with potent, selective and non-competitive, ABCG2 inhibitors was also significantly altered upon mutation. These results suggest an important mechanistic role for the C2-sequence of the ABCG2 linker region in ATP binding and/or hydrolysis coupled to drug efflux.







Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- MDR:
-
Multidrug resistance
References
Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M (1998) A human placenta-specific ATP-binding gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58(23):5337–5339
Doyle LA, Yang W, Abruzzo LW, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95(26):15665–15670
Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE (1999) Molecular cloning of cDNAs which are highly expressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59(1):152–162
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455(1):8–13
Ueda K, Clark DP, Chen CJ, Roninson IB, Gottesman MM, Pastan I (1987) The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem 262(2):505–508
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258(5088):1650–1654
Jonker JW, Buitlelaar M, Wagenaar E, van de Valk MEA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH (2002) The breasr cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 99(24):15649–15654
Sarkadi B, Homolya L, Szakacs G, Varadi A (2006) Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86(4):1179–1236
Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP (2002) Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA 99(19):12339–12344
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G (2009) Structure of P-glycoprotein reveals a molecular basis for ply-specific drug binding. Science 323(5922):1718–1722. doi:10.1126/science.1168750
Jin MS, Oldham ML, Chen J (2012) Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 490(7421):566–569. doi:10.1038/nature.11448
Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443(7108):180–185
Bakos E, Klein I, Welker E, Szabo K, Muller M, Sarkadi B, Varadi A (1997) Characterization of the human multidrug resistance protein containing mutations in the ATP-binding cassette signature region. Biochem J 323(Pt 3):777–783
Tombline G, Bartholomew L, Gimi K, Tyndall GA, Senior AE (2003) Synergy between conserved ABC signature Ser residues in P-glycoprotein catalysis. J Biol Chem 279(7):5363–5373
Szentpetery Z, Kern A, Liliom K, Sarkadi B, Varadi A, Bakos E (2004) The role of the conserved glycines of ATP-binding cassette signature motifs of MRP1 in the communication between the substrate-binding site and the catalytic centers. J Biol Chem 279(40):41670–41678
Hewitt EW, Lehrer PJ (2003) The ABC-transporter signature motif is required for peptide translocation but not peptide binding by TAP. Eur J Immunol 33(2):422–427
Chen M, Abele R, Tampé R (2004) Functional non-equivalence of ATP-binding cassette signature motifs in the transporter associated with antigen processing (TAP). J Biol Chem 279(44):46073–46081
Schmees G, Stein A, Hunke S, Landmesser H, Schneider E (1999) Functional consequences of mutations in the conserved “signature sequence” of the ATP-binding cassette protein MalK. Eur J Biochem 266(2):420–430
Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L (2005) H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J 24(11):1901–1910
The UniProt Consortium (2015) UniProt: a hub for protein information. Nucleic Acids Res 43:D204–D212
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) ClustalW and ClustalX version 2. Bioinformatics 23(21):2947–2948
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16(6):276–277
Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337:635–645
Dosztányi Z, Csizmók V, Tompa P, Simon I (2005) The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 347:827–839
Cole C, Barber JD, Barton GJ (2008) The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36:W197–W201
Buchan DWA, Minneci F, Nugent TCO, Bryson K, Jones DT (2013) Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res 41:W340–W348
Valdameri G, Genoux-Bastide E, Peres E, Gauthier C, Guitton J, Terreux R, Winnischofer SM, Rocha ME, Boumendjel A, Di Pietro A (2012) Substituted chromones as highly potent nontoxic inhibitors, specific for the breast cancer resistance protein. J Med Chem 55(2):966–970. doi:10.1021/jm201404w
Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, Sarkadi B, Bates SE (2005) Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol 56(2):161–172
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Shukla S, Robey RW, Bates SE, Ambudkar SV (2006) The calcium-channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linkedABC drug transporter, ABCG2. Biochemistry 45(29):8940–8951
Shukla S, Skoumbourdis AP, Walsh MJ, Hartz AM, Fung KL, Wu CP, Gottesman MM, Bauer B, Thomas CJ, Ambudkar SV (2011) Synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna (nilotinib): evidence for transport of Tasigna and its fluorescent derivative by ABC drug transporters. Mol Pharm 8(4):1292–1302. doi:10.1021/mp2001022
Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1(6):417–425
Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B (2004) High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol 65(6):1485–1495
Winter E, Lecerf-Schmidt F, Jabor Gozzi G, Peres B, Lightbody M, Gauthier C, Ozvegy-Laczka C, Sarkadi B, Creczynski-Pasa TB, Boumendjel A, Di Pietro A (2013) Structure-activity relationships of chromone derivatives toward mechanism of interaction with, and inhibition of, breast cancer resistance protein ABCG2. J Med Chem 56(24):9849–9860. doi:10.1021/jm401649j
Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR Jr, Chen X, Chan ZS (2009) Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol 78(2):153–161. doi:10.1016/j.bcp.2009.04.002
Hazai E, Bikadi Z (2008) Homology modeling of breast cancer resistance protein (ABCG2). J Struct Biol 162(1):63–74. doi:10.1016/jsb.2007.12.001
Al-Shawi MK, Polar MK, Omote H, Figler RA (2003) Transition state analysis of the coupling of drug transport to ATP hydrolysis by P-glycoprotein. J Biol Chem 278(52):52629–52640
Acknowledgments
Drs. A. Ahmed-Belkacem and C. Gauthier are acknowledged for their help in initiating the studies and performing some experiments, respectively. This work was supported by the CNRS and University Lyon 1 (UMR 5086), and the Ligue Nationale contre le Cancer (Equipe Labellisée Ligue 2014) to A.D.P. S.M. was recipient of fellowships from the Ligue de la Loire contre le Cancer, the Association pour la Recherche sur le Cancer and the Région Rhône-Alpes (Explora’Doc mobility program with S.E.B.). R.W.R., S.S., S.V.A. and S.E.B. were supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (Z01 BC010030-17). The authors also thank research funding from OTKA K 111678 and the Bolyai Fellowship of the Hungarian Academy of Sciences to T.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macalou, S., Robey, R.W., Jabor Gozzi, G. et al. The linker region of breast cancer resistance protein ABCG2 is critical for coupling of ATP-dependent drug transport. Cell. Mol. Life Sci. 73, 1927–1937 (2016). https://doi.org/10.1007/s00018-015-2118-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2118-5