Abstract
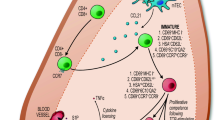
Thymic B cells are a unique population of B lymphocytes that reside at the cortico-medullary junction of the thymus, an organ that is specialized for the development and selection of T cells. These B cells are distinct from peripheral B cells both in terms of their origin and phenotype. Multiple lines of evidence suggest that they develop within the thymus from B lineage-committed progenitors and are not recirculating peripheral B cells. Furthermore, thymic B cells have a highly activated phenotype. Because of their location in the thymic medulla, they have been thought to play a role in T cell negative selection. Thymic B cells are capable of inducing negative selection in a number of model antigen systems, including viral super antigen, peptides from immunoglobulin, and cognate self antigen presented by B cell receptor-mediated uptake. These findings establish thymic B cells as a novel and important population to study; however, much work remains to be done to understand how all of these unique aspects of thymic B cell biology inform their function.


Similar content being viewed by others
References
Isaacson PG, Norton AJ, Addis BJ (1987) The human thymus contains a novel population of B lymphocytes. Lancet 2:1488–1491
Miyama-Inaba M, Kuma S, Inaba K, Ogata H, Iwai H, Yasumizu R, Muramatsu S, Steinman RM, Ikehara S (1988) Unusual phenotype of B cells in the thymus of normal mice. J Exp Med 168:811–816
Nango K, Inaba M, Inaba K, Adachi Y, Than S, Ishida T, Kumamoto T, Uyama M, Ikehara S (1991) Ontogeny of thymic B cells in normal mice. Cell Immunol 133:109–115
Akashi K, Richie LI, Miyamoto T, Carr WH, Weissman IL (2000) B lymphopoiesis in the thymus. J Immunol 164:5221–5226
Perera J, Meng L, Meng F, Huang H (2013) Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc Natl Acad Sci USA 110:17011–17016. doi:10.1073/pnas.1313001110
Than S, Inaba M, Inaba K, Fukuba Y, Adachi Y, Ikehara S (1992) Origin of thymic and peritoneal Ly-1 B cells. Eur J Immunol 22:1299–1303. doi:10.1002/eji.1830220527
Mori S, Inaba M, Sugihara A, Taketani S, Doi H, Fukuba Y, Yamamoto Y, Adachi Y, Inaba K, Fukuhara S, Ikehara S (1997) Presence of B cell progenitors in the thymus. J Immunol 158:4193–4199. doi:10.3109/10837450.2013.829099
McKenna HJ, Morrissey PJ (1998) Flt3 ligand plus IL-7 supports the expansion of murine thymic B cell progenitors that can mature intrathymically. J Immunol 160:4801–4809
Sugihara A, Inaba M, Mori SI, Taketani S, Adachi Y, Hisha H, Inaba K, Toki J, Horio T, Gershwin ME, Ikehara S (2000) Differentiation from thymic B cell progenitors to mature B cells in vitro. Immunobiology 201:515–526. doi:10.1016/S0171-2985(00)80071-6
Ceredig R (2002) The ontogeny of B cells in the thymus of normal, CD3 epsilon knockout (KO), RAG-2 KO and IL-7 transgenic mice. Int Immunol 14:87–99
Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10:547–558
Wilson A, MacDonald HR, Radtke F (2001) Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med 194:1003–1012
Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, Gossler A, Gale NW, Radtke F, Fehling HJ, Rodewald H-R (2009) Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity 30:67–79. doi:10.1016/j.immuni.2008.10.016
Goetz CA, Harmon IR, O’Neil JJ, Burchill MA, Johanns TM, Farrar MA (2005) Restricted STAT5 activation dictates appropriate thymic B versus T cell lineage commitment. J Immunol 174:7753–7763
Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, Smale ST (2013) Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol 14:1073–1083. doi:10.1038/ni.2707
Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC (2007) Commitment and developmental potential of extrathymic and intrathymic t cell precursors: plenty to choose from. Immunity 26:678–689. doi:10.1016/j.immuni.2007.05.009
Zediak VP, Maillard I, Bhandoola A (2005) Closer to the source: notch and the nature of thymus-settling cells. Immunity 23:245–248. doi:10.1016/j.immuni.2005.08.008
Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT (2004) Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity 20:735–745. doi:10.1016/j.immuni.2004.05.004
Benz C, Bleul CC (2005) A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med 202:21–31. doi:10.1084/jem.20050146
Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A (2005) Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol 6:663–670. doi:10.1038/ni1216
Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A (2009) CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood 115:1897–1905. doi:10.1182/blood-2009-08-237784
Krueger A, von Boehmer H (2007) Identification of a T lineage-committed progenitor in adult blood. Immunity 26:105–116. doi:10.1016/j.immuni.2006.12.004
Martin CH, Aifantis I, Scimone ML, von Andrian UH, Reizis B, von Boehmer H, Gounari F (2003) Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol 4:866–873. doi:10.1038/ni965
Kingston R, Jenkinson EJ, Owen JJ (1985) A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. Nature 317:811–813
Inaba M, Inaba K, Adachi Y, Nango K, Ogata H, Muramatsu S, Ikehara S (1990) Functional analyses of thymic CD5+ B cells. Responsiveness to major histocompatibility complex class II-restricted T blasts but not to lipopolysaccharide or anti-IgM plus interleukin 4. J Exp Med 171:321–326
Ferrero I, Anjuère F, Martín P, Martínez del Hoyo G, Fraga ML, Wright N, Varona R, Márquez G, Ardavín C (1999) Functional and phenotypic analysis of thymic B cells: role in the induction of T cell negative selection. Eur J Immunol 29:1598–1609. doi:10.1002/(SICI)1521-4141(199905)29:05<1598:AID-IMMU1598>3.0.CO;2-O
Inaba M, Inaba K, Fukuba Y, Mori S, Haruna H, Doi H, Adachi Y, Iwai H, Hosaka N, Hisha H (1995) Activation of thymic B cells by signals of CD40 molecules plus interleukin-10. Eur J Immunol 25:1244–1248. doi:10.1002/eji.1830250517
Akirav EM, Xu Y, Ruddle NH (2011) Resident B cells regulate thymic expression of myelin oligodendrocyte glycoprotein. J Neuroimmunol 235:33–39. doi:10.1016/j.jneuroim.2011.03.013
Fujihara C, Williams JA, Watanabe M, Jeon H, Sharrow SO, Hodes RJ (2014) T Cell-B cell thymic cross-talk: maintenance and function of thymic B Cells requires cognate CD40-CD40 ligand interaction. J Immunol 193:5534–5544. doi:10.4049/jimmunol.1401655
Kantor AB, Herzenberg LA (1993) Origin of murine B cell lineages. Annu Rev Immunol 11:501–538. doi:10.1146/annurev.iy.11.040193.002441
Kantor AB, Stall AM, Adams S, Herzenberg LA (1992) Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA 89:3320–3324
Wortis HH, Teutsch M, Higer M, Zheng J, Parker DC (1995) B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA 92:3348–3352
van Ewijk W (1984) Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat 170:311–330. doi:10.1002/aja.1001700307
Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA (2009) The impact of negative selection on thymocyte migration in the medulla. Nat Immunol 10:823–830. doi:10.1038/ni.1761
Wu L, Shortman K (2005) Heterogeneity of thymic dendritic cells. Semin Immunol 17:304–312. doi:10.1016/j.smim.2005.05.001
Inaba M, Inaba K, Hosono M, Kumamoto T, Ishida T, Muramatsu S, Masuda T, Ikehara S (1991) Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med 173:549–559
Mazda O, Watanabe Y, Gyotoku J, Katsura Y (1991) Requirement of dendritic cells and B cells in the clonal deletion of Mls-reactive T cells in the thymus. J Exp Med 173:539–547
Molina IJ, Cannon NA, Hyman R, Huber BT (1989) Macrophages and T cells do not express Mlsa determinants. J Immunol 143:39–44
Ahmed A, Scher I, Smith AH, Sell KW (1977) Studies on non-H-2 linked lymphocyte activating determinants. I. Description of the cell type bearing the Mls product. J Immunogenet 4:201–213
Kleindienst P, Chretien I, Winkler T, Brocker T (2000) Functional comparison of thymic B cells and dendritic cells in vivo. Blood 95:2610–2616
Frommer F, Waisman A (2010) B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS One 5:e15372. doi:10.1371/journal.pone.0015372
Morlacchi S, Soldani C, Viola A, Sarukhan A (2011) Self-antigen presentation by mouse B cells results in regulatory T-cell induction rather than anergy or clonal deletion. Blood 118:984–991. doi:10.1182/blood-2011-02-336115
Detanico T, Heiser RA, Aviszus K, Bonorino C, Wysocki LJ (2011) Self-tolerance checkpoints in CD4 T cells specific for a peptide derived from the B cell antigen receptor. J Immunol 187:82–91. doi:10.4049/jimmunol.1002287
Rudensky AY, Mazel SM, Yurin VL (1990) Presentation of endogenous immunoglobulin determinant to immunoglobulin-recognizing T cell clones by the thymic cells. Eur J Immunol 20:2235–2239. doi:10.1002/eji.1830201012
Zöller M (1990) Intrathymic presentation of nominal antigen by B cells. Int Immunol 2:427–434
Werner-Klein M, Dresch C, Marconi P, Brocker T (2007) Transcriptional targeting of B cells for induction of peripheral CD8 T cell tolerance. J Immunol 178:7738–7746
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perera, J., Huang, H. The development and function of thymic B cells. Cell. Mol. Life Sci. 72, 2657–2663 (2015). https://doi.org/10.1007/s00018-015-1895-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1895-1