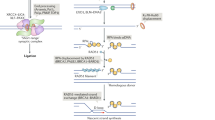
Abstract
Exogenous and endogenous genotoxic agents, such as ionizing radiation and numerous chemical agents, cause DNA double-strand breaks (DSBs), which are highly toxic and lead to genomic instability or tumorigenesis if not repaired accurately and efficiently. Cells have over evolutionary time developed certain repair mechanisms in response to DSBs to maintain genomic integrity. Major DSB repair mechanisms include non-homologous end joining and homologous recombination (HR). Using sister homologues as templates, HR is a high-fidelity repair pathway that can rejoin DSBs without introducing mutations. However, HR execution without appropriate guarding may lead to more severe gross genome rearrangements. Here we review current knowledge regarding the factors and mechanisms required for accomplishment of accurate HR.

Similar content being viewed by others
References
van Gent DC, Hoeijmakers JH, Kanaar R (2001) Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet 2(3):196–206. doi:10.1038/35056049
Shiloh Y, Lehmann AR (2004) Maintaining integrity. Nat Cell Biol 6(10):923–928. doi:10.1038/ncb1004-923
Schar P (2001) Spontaneous DNA damage, genome instability, and cancer–when DNA replication escapes control. Cell 104(3):329–332 (pii: S0092-8674(01)00220-3)
Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, Sleckman BP (2006) ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature 442(7101):466–470. doi:10.1038/nature04866
Huang CY, Sharma GG, Walker LM, Bassing CH, Pandita TK, Sleckman BP (2007) Defects in coding joint formation in vivo in developing ATM-deficient B and T lymphocytes. J Exp Med 204(6):1371–1381. doi:10.1084/jem.20061460
Malu S, Malshetty V, Francis D, Cortes P (2012) Role of non-homologous end joining in V(D)J recombination. Immunol Res 54(1–3):233–246. doi:10.1007/s12026-012-8329-z
Alt FW, Zhang Y, Meng FL, Guo C, Schwer B (2013) Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell 152(3):417–429
Setlow RB (1978) Repair deficient human disorders and cancer. Nature 271(5647):713–717
Hakem R (2008) DNA-damage repair; the good, the bad, and the ugly. EMBO J 27(4):589–605. doi:10.1038/emboj.2008.15emboj200815
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461(7267):1071–1078. doi:10.1038/nature08467nature08467
San Filippo J, Sung P, Klein H (2008) Mechanism of eukaryotic homologous recombination. Annu Rev Biochem 77:229–257. doi:10.1146/annurev.biochem.77.061306.125255
Lieber MR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79:181–211. doi:10.1146/annurev.biochem.052308.093131
Heyer WD, Ehmsen KT, Liu J (2010) Regulation of homologous recombination in eukaryotes. Annu Rev Genet 44:113–139. doi:10.1146/annurev-genet-051710-150955
Betermier M, Bertrand P, Lopez BS (2014) Is non-homologous end-joining really an inherently error-prone process? PLoS Genet 10(1):e1004086. doi:10.1371/journal.pgen.1004086
Hicks WM, Kim M, Haber JE (2010) Increased mutagenesis and unique mutation signature associated with mitotic gene conversion. Science 329(5987):82–85. doi:10.1126/science.1191125
Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkova A (2011) Break-induced replication is highly inaccurate. PLoS Biol 9(2):e1000594. doi:10.1371/journal.pbio.1000594
Iraqui I, Chekkal Y, Jmari N, Pietrobon V, Freon K, Costes A, Lambert SA (2012) Recovery of arrested replication forks by homologous recombination is error-prone. PLoS Genet 8(10):e1002976. doi:10.1371/journal.pgen.1002976
Mizuno K, Miyabe I, Schalbetter SA, Carr AM, Murray JM (2013) Recombination-restarted replication makes inverted chromosome fusions at inverted repeats. Nature 493(7431):246–249. doi:10.1038/nature11676nature11676
Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S (2006) Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst) 5(9–10):1021–1029. doi:10.1016/j.dnarep.2006.05.022
Richardson C, Jasin M (2000) Coupled homologous and nonhomologous repair of a double-strand break preserves genomic integrity in mammalian cells. Mol Cell Biol 20(23):9068–9075
Kasparek TR, Humphrey TC (2011) DNA double-strand break repair pathways, chromosomal rearrangements and cancer. Semin Cell Dev Biol 22(8):886–897. doi:10.1016/j.semcdb.2011.10.007
Daley JM, Sung P (2014) 53BP1, BRCA1 and the choice between recombination and end joining at DNA double-strand breaks. Mol Cell Biol. doi:10.1128/MCB.01639-13
Branzei D, Foiani M (2008) Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol 9(4):297–308. doi:10.1038/nrm2351nrm2351
Brandsma I, Gent DC (2012) Pathway choice in DNA double strand break repair: observations of a balancing act. Genome Integr 3(1):9. doi:10.1186/2041-9414-3-9
Chapman JR, Taylor MR, Boulton SJ (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47(4):497–510. doi:10.1016/j.molcel.2012.07.029
Karanam K, Kafri R, Loewer A, Lahav G (2012) Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. Mol Cell 47(2):320–329. doi:10.1016/j.molcel.2012.05.052
Rothkamm K, Kruger I, Thompson LH, Lobrich M (2003) Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 23(16):5706–5715
Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45:247–271. doi:10.1146/annurev-genet-110410-132435
West SC (2003) Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol 4(6):435–445. doi:10.1038/nrm1127
Ciccia A, Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40(2):179–204. doi:10.1016/j.molcel.2010.09.019
Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng CX, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A (2010) 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141(2):243–254. doi:10.1016/j.cell.2010.03.012
Davis AJ, Chen DJ (2013) DNA double strand break repair via non-homologous end-joining. Transl Cancer Res 2(3):130–143. doi:10.3978/j.issn.2218-676X.2013.04.02
Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, Mizuta R, Varghese AJ, Alt FW, Jeggo PA, Jackson SP (1995) Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80(5):813–823 (pii 0092-8674(95)90360-7)
Sipley JD, Menninger JC, Hartley KO, Ward DC, Jackson SP, Anderson CW (1995) Gene for the catalytic subunit of the human DNA-activated protein kinase maps to the site of the XRCC7 gene on chromosome 8. Proc Natl Acad Sci USA 92(16):7515–7519
Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, Jackson SP (1995) DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell 82(5):849–856 (pii 0092-8674(95)90482-4)
Ma Y, Pannicke U, Schwarz K, Lieber MR (2002) Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108(6):781–794 pii S0092867402006712
Ma Y, Schwarz K, Lieber MR (2005) The Artemis:DNA-PKcs endonuclease cleaves DNA loops, flaps, and gaps. DNA Repair (Amst) 4(7):845–851. doi:10.1016/j.dnarep.2005.04.013
Goodarzi AA, Yu Y, Riballo E, Douglas P, Walker SA, Ye R, Harer C, Marchetti C, Morrice N, Jeggo PA, Lees-Miller SP (2006) DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J 25(16):3880–3889. doi:10.1038/sj.emboj.7601255
van der Burg M, van Dongen JJ, van Gent DC (2009) DNA-PKcs deficiency in human: long predicted, finally found. Curr Opin Allergy Clin Immunol 9(6):503–509. doi:10.1097/ACI.0b013e3283327e41
Teo SH, Jackson SP (1997) Identification of Saccharomyces cerevisiae DNA ligase IV: involvement in DNA double-strand break repair. EMBO J 16(15):4788–4795. doi:10.1093/emboj/16.15.4788
Schar P, Herrmann G, Daly G, Lindahl T (1997) A newly identified DNA ligase of Saccharomyces cerevisiae involved in RAD52-independent repair of DNA double-strand breaks. Genes Dev 11(15):1912–1924
Wilson TE, Grawunder U, Lieber MR (1997) Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature 388(6641):495–498. doi:10.1038/41365
Critchlow SE, Bowater RP, Jackson SP (1997) Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Curr Biol 7(8):588–598 pii: S0960-9822(06)00258-2
Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR (1998) DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell 2(4):477–484 (pii S1097-2765(00)80147-1)
Grawunder U, Zimmer D, Leiber MR (1998) DNA ligase IV binds to XRCC4 via a motif located between rather than within its BRCT domains. Curr Biol 8(15):873–876 (pii S0960-9822(07)00349-1)
Ahnesorg P, Smith P, Jackson SP (2006) XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell 124(2):301–313. doi:10.1016/j.cell.2005.12.031
Zha S, Alt FW, Cheng HL, Brush JW, Li G (2007) Defective DNA repair and increased genomic instability in Cernunnos-XLF-deficient murine ES cells. Proc Natl Acad Sci USA 104(11):4518–4523. doi:10.1073/pnas.0611734104
Andres SN, Modesti M, Tsai CJ, Chu G, Junop MS (2007) Crystal structure of human XLF: a twist in nonhomologous DNA end-joining. Mol Cell 28(6):1093–1101. doi:10.1016/j.molcel.2007.10.024
Li Y, Chirgadze DY, Bolanos-Garcia VM, Sibanda BL, Davies OR, Ahnesorg P, Jackson SP, Blundell TL (2008) Crystal structure of human XLF/Cernunnos reveals unexpected differences from XRCC4 with implications for NHEJ. EMBO J 27(1):290–300. doi:10.1038/sj.emboj.7601942
Chistiakov DA, Voronova NV, Chistiakov AP (2009) Ligase IV syndrome. Eur J Med Genet 52(6):373–378. doi:10.1016/j.ejmg.2009.05.009
Frit P, Barboule N, Yuan Y, Gomez D, Calsou P (2014) Alternative end-joining pathway(s): bricolage at DNA breaks. DNA Repair (Amst). doi:10.1016/j.dnarep.2014.02.007
Mine-Hattab J, Rothstein R (2012) Increased chromosome mobility facilitates homology search during recombination. Nat Cell Biol 14(5):510–517. doi:10.1038/ncb2472ncb2472
Dion V, Kalck V, Horigome C, Towbin BD, Gasser SM (2012) Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat Cell Biol 14(5):502–509. doi:10.1038/ncb2465
Sung P, Klein H (2006) Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol 7(10):739–750. doi:10.10/nrm2008
Bordeianu G, Zugun-Eloae F, Rusu MG (2011) The role of DNA repair by homologous recombination in oncogenesis. Rev Med Chir Soc Med Nat Iasi 115(4):1189–1194
Kakarougkas A, Jeggo PA (2014) DNA DSB repair pathway choice: an orchestrated handover mechanism. Br J Radiol. doi:10.1259/bjr.20130685
Barlow JH, Lisby M, Rothstein R (2008) Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell 30(1):73–85. doi:10.1016/j.molcel.2008.01.016
Yu X, Baer R (2000) Nuclear localization and cell cycle-specific expression of CtIP, a protein that associates with the BRCA1 tumor suppressor. J Biol Chem 275(24):18541–18549. doi:10.1074/jbc.M909494199
Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP (2008) CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 455(7213):689–692. doi:10.1038/nature07215
Langerak P, Russell P (2011) Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Philos Trans R Soc Lond B Biol Sci 366(1584):3562–3571. doi:10.1098/rstb.2011.0070
Huertas P, Jackson SP (2009) Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem 284(14):9558–9565. doi:10.1074/jbc.M808906200
Panier S, Boulton SJ (2014) Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 15(1):7–18. doi:10.1038/nrm3719
Longhese MP, Bonetti D, Manfrini N, Clerici M (2010) Mechanisms and regulation of DNA end resection. EMBO J 29(17):2864–2874. doi:10.1038/emboj.2010.165
Mimitou EP, Symington LS (2011) DNA end resection–unraveling the tail. DNA Repair (Amst) 10(3):344–348. doi:10.1016/j.dnarep.2010.12.004
Huertas P (2010) DNA resection in eukaryotes: deciding how to fix the break. Nat Struct Mol Biol 17(1):11–16. doi:10.1038/nsmb.1710
Mimitou EP, Symington LS (2009) DNA end resection: many nucleases make light work. DNA Repair (Amst) 8(9):983–995. doi:10.1016/j.dnarep.2009.04.017
Ohta K, Nicolas A, Furuse M, Nabetani A, Ogawa H, Shibata T (1998) Mutations in the MRE11, RAD50, XRS2, and MRE2 genes alter chromatin configuration at meiotic DNA double-stranded break sites in premeiotic and meiotic cells. Proc Natl Acad Sci USA 95(2):646–651
D’Amours D, Jackson SP (2002) The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat Rev Mol Cell Biol 3(5):317–327. doi:10.1038/nrm805nrm805
Trujillo KM, Roh DH, Chen L, Van Komen S, Tomkinson A, Sung P (2003) Yeast xrs2 binds DNA and helps target rad50 and mre11 to DNA ends. J Biol Chem 278(49):48957–48964. doi:10.1074/jbc.M309877200
Lukas C, Melander F, Stucki M, Falck J, Bekker-Jensen S, Goldberg M, Lerenthal Y, Jackson SP, Bartek J, Lukas J (2004) Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J 23(13):2674–2683. doi:10.1038/sj.emboj.7600269
Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA (2001) Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell 105(4):473–485 (pii: S0092-8674(01)00335-X)
Costanzo V, Robertson K, Bibikova M, Kim E, Grieco D, Gottesman M, Carroll D, Gautier J (2001) Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol Cell 8(1):137–147 (pii:S1097-2765(01)00294-5)
Falck J, Petrini JH, Williams BR, Lukas J, Bartek J (2002) The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet 30(3):290–294. doi:10.1038/ng845ng845
Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y (2003) Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 22(20):5612–5621. doi:10.1093/emboj/cdg541
Lee JH, Paull TT (2004) Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304(5667):93–96. doi:10.1126/science.1091496
Sun Y, Jiang X, Xu Y, Ayrapetov MK, Moreau LA, Whetstine JR, Price BD (2009) Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol 11(11):1376–1382. doi:10.1038/ncb1982
Chailleux C, Tyteca S, Papin C, Boudsocq F, Puget N, Courilleau C, Grigoriev M, Canitrot Y, Trouche D (2010) Physical interaction between the histone acetyl transferase Tip60 and the DNA double-strand breaks sensor MRN complex. Biochem J 426(3):365–371. doi:10.1042/BJ20091329
Lee JH, Goodarzi AA, Jeggo PA, Paull TT (2010) 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J 29(3):574–585. doi:10.1038/emboj.2009.372
Yuan J, Chen J (2010) MRE11-RAD50-NBS1 complex dictates DNA repair independent of H2AX. J Biol Chem 285(2):1097–1104. doi:10.1074/jbc.M109.078436
Grenon M, Gilbert C, Lowndes NF (2001) Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat Cell Biol 3(9):844–847. doi:10.1038/ncb0901-844
Lee JH, Paull TT (2005) ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308(5721):551–554. doi:10.1126/science.1108297
Haber JE (1998) The many interfaces of Mre11. Cell 95(5):583–586 (pii: S0092-8674(00)81626-8)
Paull TT, Gellert M (1998) The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell 1(7):969–979 (pii: S1097-2765(00)80097-0)
Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K (1998) Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J 17(21):6412–6425. doi:10.1093/emboj/17.21.6412
van den Bosch M, Bree RT, Lowndes NF (2003) The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep 4(9):844–849. doi:10.1038/sj.embor.embor925
Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, Moiani D, Carney JP, Russell P, Tainer JA (2008) Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell 135(1):97–109. doi:10.1016/j.cell.2008.08.017
Buis J, Wu Y, Deng Y, Leddon J, Westfield G, Eckersdorff M, Sekiguchi JM, Chang S, Ferguson DO (2008) Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell 135(1):85–96. doi:10.1016/j.cell.2008.08.015
Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, Alt FW, Petrini JH (2003) Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell 12(6):1511–1523 pii: S1097276503004556
Stracker TH, Petrini JH (2011) The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 12(2):90–103. doi:10.1038/nrm3047nrm3047
Mimitou EP, Symington LS (2008) Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455(7214):770–774. doi:10.1038/nature07312
Shibata A, Moiani D, Arvai AS, Perry J, Harding SM, Genois MM, Maity R, van Rossum-Fikkert S, Kertokalio A, Romoli F, Ismail A, Ismalaj E, Petricci E, Neale MJ, Bristow RG, Masson JY, Wyman C, Jeggo PA, Tainer JA (2014) DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol Cell 53(1):7–18. doi:10.1016/j.molcel.2013.11.003
Dong Z, Zhong Q, Chen PL (1999) The Nijmegen breakage syndrome protein is essential for Mre11 phosphorylation upon DNA damage. J Biol Chem 274(28):19513–19516
Paull TT, Gellert M (1999) Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev 13(10):1276–1288
Luo G, Yao MS, Bender CF, Mills M, Bladl AR, Bradley A, Petrini JH (1999) Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc Natl Acad Sci USA 96(13):7376–7381
Kang J, Bronson RT, Xu Y (2002) Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J 21(6):1447–1455. doi:10.1093/emboj/21.6.1447
Williams BR, Mirzoeva OK, Morgan WF, Lin J, Dunnick W, Petrini JH (2002) A murine model of Nijmegen breakage syndrome. Curr Biol 12(8):648–653 (pii: S0960982202007637)
Zhu J, Petersen S, Tessarollo L, Nussenzweig A (2001) Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr Biol 11(2):105–109 (pii: S0960-9822(01)00019-7)
Tauchi H, Kobayashi J, Morishima K, van Gent DC, Shiraishi T, Verkaik NS, vanHeems D, Ito E, Nakamura A, Sonoda E, Takata M, Takeda S, Matsuura S, Komatsu K (2002) Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature 420(6911):93–98. doi:10.1038/nature01125
Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH (2007) The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature 447(7141):218–221. doi:10.1038/nature05740
Williams RS, Dodson GE, Limbo O, Yamada Y, Williams JS, Guenther G, Classen S, Glover JN, Iwasaki H, Russell P, Tainer JA (2009) Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell 139(1):87–99. doi:10.1016/j.cell.2009.07.033
Hopfner KP, Tainer JA (2003) Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr Opin Struct Biol 13(2):249–255 (pii: S0959440X0300037X)
van Noort J, van Der Heijden T, de Jager M, Wyman C, Kanaar R, Dekker C (2003) The coiled-coil of the human Rad50 DNA repair protein contains specific segments of increased flexibility. Proc Natl Acad Sci USA 100(13):7581–7586. doi:10.1073/pnas.1330706100
Lichten M (2005) Rad50 connects by hook or by crook. Nat Struct Mol Biol 12(5):392–393. doi:10.1038/nsmb0505-392
Paull TT (2001) New glimpses of an old machine. Cell 107(5):563–565 (pii: S0092-8674(01)00591-8)
de Jager M, van Noort J, van Gent DC, Dekker C, Kanaar R, Wyman C (2001) Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol Cell 8(5):1129–1135 (pii: S1097-2765(01)00381-1)
Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karcher A, Henderson B, Bodmer JL, McMurray CT, Carney JP, Petrini JH, Tainer JA (2002) The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418(6897):562–566. doi:10.1038/nature00922
Schaeper U, Subramanian T, Lim L, Boyd JM, Chinnadurai G (1998) Interaction between a cellular protein that binds to the C-terminal region of adenovirus E1A (CtBP) and a novel cellular protein is disrupted by E1A through a conserved PLDLS motif. J Biol Chem 273(15):8549–8552
Yu X, Wu LC, Bowcock AM, Aronheim A, Baer R (1998) The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J Biol Chem 273(39):25388–25392
Wong AK, Ormonde PA, Pero R, Chen Y, Lian L, Salada G, Berry S, Lawrence Q, Dayananth P, Ha P, Tavtigian SV, Teng DH, Bartel PL (1998) Characterization of a carboxy-terminal BRCA1 interacting protein. Oncogene 17(18):2279–2285. doi:10.1038/sj.onc.1202150
You Z, Shi LZ, Zhu Q, Wu P, Zhang YW, Basilio A, Tonnu N, Verma IM, Berns MW, Hunter T (2009) CtIP links DNA double-strand break sensing to resection. Mol Cell 36(6):954–969. doi:10.1016/j.molcel.2009.12.002
Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP (2007) Human CtIP promotes DNA end resection. Nature 450(7169):509–514. doi:10.1038/nature06337
Takeda S, Nakamura K, Taniguchi Y, Paull TT (2007) Ctp1/CtIP and the MRN complex collaborate in the initial steps of homologous recombination. Mol Cell 28(3):351–352. doi:10.1016/j.molcel.2007.10.016
Limbo O, Chahwan C, Yamada Y, de Bruin RA, Wittenberg C, Russell P (2007) Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol Cell 28(1):134–146. doi:10.1016/j.molcel.2007.09.009
Chen L, Nievera CJ, Lee AY, Wu X (2008) Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem 283(12):7713–7720. doi:10.1074/jbc.M710245200
Tsukuda T, Fleming AB, Nickoloff JA, Osley MA (2005) Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature 438(7066):379–383. doi:10.1038/nature04148
Liang B, Qiu J, Ratnakumar K, Laurent BC (2007) RSC functions as an early double-strand-break sensor in the cell’s response to DNA damage. Curr Biol 17(16):1432–1437. doi:10.1016/j.cub.2007.07.035
Yeeles JT, Dillingham MS (2010) The processing of double-stranded DNA breaks for recombinational repair by helicase-nuclease complexes. DNA Repair (Amst) 9(3):276–285. doi:10.1016/j.dnarep.2009.12.016
Szankasi P, Smith GR (1995) A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction. Science 267(5201):1166–1169
Lieber MR (1997) The FEN-1 family of structure-specific nucleases in eukaryotic DNA replication, recombination and repair. BioEssays 19(3):233–240. doi:10.1002/bies.950190309
Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, Kolodner RD (1997) Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci USA 94(14):7487–7492
Tsubouchi H, Ogawa H (2000) Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol Biol Cell 11(7):2221–2233
Zhu Z, Chung WH, Shim EY, Lee SE, Ira G (2008) Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134(6):981–994. doi:10.1016/j.cell.2008.08.037
Symington LS (2002) Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev 66(4):630–670 (table of contents)
Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC (2008) Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A 105(44):16906–16911. doi:10.1073/pnas.08093801050809380105
Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, Ira G, Sung P (2010) Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 467(7311):108–111. doi:10.1038/nature09318nature09318
Chen H, Symington LS (2013) Overcoming the chromatin barrier to end resection. Cell Res 23(3):317–319. doi:10.1038/cr.2012.148
Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, Koshland D (2004) DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol Cell 16(6):991–1002. doi:10.1016/j.molcel.2004.11.027
van Attikum H, Gasser SM (2005) The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol 6(10):757–765. doi:10.1038/nrm1737
Altaf M, Saksouk N, Cote J (2007) Histone modifications in response to DNA damage. Mutat Res 618(1–2):81–90. doi:10.1016/j.mrfmmm.2006.09.009
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273(10):5858–5868
Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR 3rd, Abmayr SM, Washburn MP, Workman JL (2004) Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306(5704):2084–2087. doi:10.1126/science.1103455
Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J (2004) Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 16(6):979–990. doi:10.1016/j.molcel.2004.12.003
Thiriet C, Hayes JJ (2005) Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell 18(6):617–622. doi:10.1016/j.molcel.2005.05.008
Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J (2005) Gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 20(5):801–809. doi:10.1016/j.molcel.2005.10.003
Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL (2006) Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311(5762):844–847. doi:10.1126/science.1124000
Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, Lieberman J, Shen X, Buratowski S, Haber JE, Durocher D, Greenblatt JF, Krogan NJ (2006) A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 439(7075):497–501. doi:10.1038/nature04384
Yuan J, Adamski R, Chen J (2010) Focus on histone variant H2AX: to be or not to be. FEBS Lett 584(17):3717–3724. doi:10.1016/j.febslet.2010.05.021
Neely KE, Hassan AH, Brown CE, Howe L, Workman JL (2002) Transcription activator interactions with multiple SWI/SNF subunits. Mol Cell Biol 22(6):1615–1625
van Attikum H, Fritsch O, Hohn B, Gasser SM (2004) Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 119(6):777–788. doi:10.1016/j.cell.2004.11.033
Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, Shen X (2004) INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119(6):767–775. doi:10.1016/j.cell.2004.11.037
Shim EY, Ma JL, Oum JH, Yanez Y, Lee SE (2005) The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks. Mol Cell Biol 25(10):3934–3944. doi:10.1128/MCB.25.10.3934-3944.2005
Shim EY, Hong SJ, Oum JH, Yanez Y, Zhang Y, Lee SE (2007) RSC mobilizes nucleosomes to improve accessibility of repair machinery to the damaged chromatin. Mol Cell Biol 27(5):1602–1613. doi:10.1128/MCB.01956-06
Chambers AL, Downs JA (2012) The RSC and INO80 chromatin-remodeling complexes in DNA double-strand break repair. Prog Mol Biol Transl Sci 110:229–261. doi:10.1016/B978-0-12-387665-2.00009-2
Costelloe T, Louge R, Tomimatsu N, Mukherjee B, Martini E, Khadaroo B, Dubois K, Wiegant WW, Thierry A, Burma S, van Attikum H, Llorente B (2012) The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489(7417):581–584. doi:10.1038/nature11353
Chen X, Cui D, Papusha A, Zhang X, Chu CD, Tang J, Chen K, Pan X, Ira G (2012) The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature 489(7417):576–580. doi:10.1038/nature11355
Eapen VV, Sugawara N, Tsabar M, Wu WH, Haber JE (2012) The Saccharomyces cerevisiae chromatin remodeler Fun30 regulates DNA end resection and checkpoint deactivation. Mol Cell Biol 32(22):4727–4740. doi:10.1128/MCB.00566-12
Lazzaro F, Sapountzi V, Granata M, Pellicioli A, Vaze M, Haber JE, Plevani P, Lydall D, Muzi-Falconi M (2008) Histone methyltransferase Dot1 and Rad9 inhibit single-stranded DNA accumulation at DSBs and uncapped telomeres. EMBO J 27(10):1502–1512. doi:10.1038/emboj.2008.81
Pandita TK, Richardson C (2009) Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res 37(5):1363–1377. doi:10.1093/nar/gkn1071
Price BD, D’Andrea AD (2013) Chromatin remodeling at DNA double-strand breaks. Cell 152(6):1344–1354. doi:10.1016/j.cell.2013.02.011
Rappold I, Iwabuchi K, Date T, Chen J (2001) Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. J Cell Biol 153(3):613–620
DiTullio RA Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, Halazonetis TD (2002) 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4(12):998–1002. doi:10.1038/ncb892
Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A (2002) DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol 4(12):993–997. doi:10.1038/ncb884
Harrison JC, Haber JE (2006) Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet 40:209–235. doi:10.1146/annurev.genet.40.051206.105231
Wang B, Matsuoka S, Carpenter PB, Elledge SJ (2002) 53BP1, a mediator of the DNA damage checkpoint. Science 298(5597):1435–1438. doi:10.1126/science.1076182182
Ward IM, Minn K, van Deursen J, Chen J (2003) p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol 23(7):2556–2563
Mochan TA, Venere M, DiTullio RA Jr, Halazonetis TD (2004) 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 3(8–9):945–952. doi:10.1016/j.dnarep.2004.03.017
Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, Hiddingh S, Thanasoula M, Kulkarni A, Yang Q, Haffty BG, Tommiska J, Blomqvist C, Drapkin R, Adams DJ, Nevanlinna H, Bartek J, Tarsounas M, Ganesan S, Jonkers J (2010) 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol 17(6):688–695. doi:10.1038/nsmb.1831nsmb.1831
Difilippantonio S, Gapud E, Wong N, Huang CY, Mahowald G, Chen HT, Kruhlak MJ, Callen E, Livak F, Nussenzweig MC, Sleckman BP, Nussenzweig A (2008) 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature 456(7221):529–533. doi:10.1038/nature07476
Dimitrova N, Chen YC, Spector DL, de Lange T (2008) 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456(7221):524–528. doi:10.1038/nature07433
Callen E, Di Virgilio M, Kruhlak MJ, Nieto-Soler M, Wong N, Chen HT, Faryabi RB, Polato F, Santos M, Starnes LM, Wesemann DR, Lee JE, Tubbs A, Sleckman BP, Daniel JA, Ge K, Alt FW, Fernandez-Capetillo O, Nussenzweig MC, Nussenzweig A (2013) 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 153(6):1266–1280. doi:10.1016/j.cell.2013.05.023
Bothmer A, Robbiani DF, Di Virgilio M, Bunting SF, Klein IA, Feldhahn N, Barlow J, Chen HT, Bosque D, Callen E, Nussenzweig A, Nussenzweig MC (2011) Regulation of DNA end joining, resection, and immunoglobulin class switch recombination by 53BP1. Mol Cell 42(3):319–329. doi:10.1016/j.molcel.2011.03.019
Li S, Chen PL, Subramanian T, Chinnadurai G, Tomlinson G, Osborne CK, Sharp ZD, Lee WH (1999) Binding of CtIP to the BRCT repeats of BRCA1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J Biol Chem 274(16):11334–11338
Wu-Baer F, Baer R (2001) Effect of DNA damage on a BRCA1 complex. Nature 414(6859):36. doi:10.1038/35102118
Yu X, Fu S, Lai M, Baer R, Chen J (2006) BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev 20(13):1721–1726. doi:10.1101/gad.1431006
Bunting SF, Callen E, Kozak ML, Kim JM, Wong N, Lopez-Contreras AJ, Ludwig T, Baer R, Faryabi RB, Malhowski A, Chen HT, Fernandez-Capetillo O, D’Andrea A, Nussenzweig A (2012) BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol Cell 46(2):125–135. doi:10.1016/j.molcel.2012.02.015
Yun MH, Hiom K (2009) CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 459(7245):460–463. doi:10.1038/nature07955
Huen MS, Sy SM, Chen J (2010) BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol 11(2):138–148. doi:10.1038/nrm2831
Reczek CR, Szabolcs M, Stark JM, Ludwig T, Baer R (2013) The interaction between CtIP and BRCA1 is not essential for resection-mediated DNA repair or tumor suppression. J Cell Biol 201(5):693–707. doi:10.1083/jcb.201302145
Cao L, Xu X, Bunting SF, Liu J, Wang RH, Cao LL, Wu JJ, Peng TN, Chen J, Nussenzweig A, Deng CX, Finkel T (2009) A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol Cell 35(4):534–541. doi:10.1016/j.molcel.2009.06.037
Gong Z, Cho YW, Kim JE, Ge K, Chen J (2009) Accumulation of Pax2 transactivation domain interaction protein (PTIP) at sites of DNA breaks via RNF8-dependent pathway is required for cell survival after DNA damage. J Biol Chem 284(11):7284–7293. doi:10.1074/jbc.M809158200
Zimmermann M, Lottersberger F, Buonomo SB, Sfeir A, de Lange T (2013) 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339(6120):700–704. doi:10.1126/science.1231573
Chapman JR, Barral P, Vannier JB, Borel V, Steger M, Tomas-Loba A, Sartori AA, Adams IR, Batista FD, Boulton SJ (2013) RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol Cell 49(5):858–871. doi:10.1016/j.molcel.2013.01.002
Escribano-Diaz C, Orthwein A, Fradet-Turcotte A, Xing M, Young JT, Tkac J, Cook MA, Rosebrock AP, Munro M, Canny MD, Xu D, Durocher D (2013) A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol Cell 49(5):872–883. doi:10.1016/j.molcel.2013.01.001
Daley JM, Sung P (2013) RIF1 in DNA break repair pathway choice. Mol Cell 49(5):840–841. doi:10.1016/j.molcel.2013.02.019
Hardy CF, Sussel L, Shore D (1992) A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev 6(5):801–814
Silverman J, Takai H, Buonomo SB, Eisenhaber F, de Lange T (2004) Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev 18(17):2108–2119. doi:10.1101/gad.121600418/17/2108
Feng L, Fong KW, Wang J, Wang W, Chen J (2013) RIF1 counteracts BRCA1-mediated end resection during DNA repair. J Biol Chem 288(16):11135–11143. doi:10.1074/jbc.M113.457440
Kumar R, Cheok CF (2014) RIF1: a novel regulatory factor for DNA replication and DNA damage response signaling. DNA Repair (Amst) 15:54–59. doi:10.1016/j.dnarep.2013.12.004
Jowsey PA, Doherty AJ, Rouse J (2004) Human PTIP facilitates ATM-mediated activation of p53 and promotes cellular resistance to ionizing radiation. J Biol Chem 279(53):55562–55569. doi:10.1074/jbc.M411021200
Wang X, Takenaka K, Takeda S (2010) PTIP promotes DNA double-strand break repair through homologous recombination. Genes Cells. doi:10.1111/j.1365-2443.2009.01379.x
Daniel JA, Santos MA, Wang Z, Zang C, Schwab KR, Jankovic M, Filsuf D, Chen HT, Gazumyan A, Yamane A, Cho YW, Sun HW, Ge K, Peng W, Nussenzweig MC, Casellas R, Dressler GR, Zhao K, Nussenzweig A (2010) PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science 329(5994):917–923. doi:10.1126/science.1187942
Munoz IM, Jowsey PA, Toth R, Rouse J (2007) Phospho-epitope binding by the BRCT domains of hPTIP controls multiple aspects of the cellular response to DNA damage. Nucleic Acids Res 35(16):5312–5322. doi:10.1093/nar/gkm493
Escribano-Diaz C, Durocher D (2013) DNA repair pathway choice–a PTIP of the hat to 53BP1. EMBO Rep 14(8):665–666. doi:10.1038/embor.2013.99embor201399
Sakaguchi K, Ishibashi T, Uchiyama Y, Iwabata K (2009) The multi-replication protein A (RPA) system–a new perspective. FEBS J 276(4):943–963. doi:10.1111/j.1742-4658.2008.06841.x
Baumann P, West SC (1998) Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci 23(7):247–251 (pii: S0968-0004(98)01232-8)
Bishop DK, Park D, Xu L, Kleckner N (1992) DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69(3):439–456 (pii: 0092-8674(92)90446-J)
Sehorn MG, Sung P (2004) Meiotic recombination: an affair of two recombinases. Cell Cycle 3(11):1375–1377
Kawabata M, Kawabata T, Nishibori M (2005) Role of recA/RAD51 family proteins in mammals. Acta Med Okayama 59(1):1–9
Shinohara A, Ogawa H, Ogawa T (1992) Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69(3):457–470 pii: 0092-8674(92)90447-K
Sehorn MG, Sigurdsson S, Bussen W, Unger VM, Sung P (2004) Human meiotic recombinase Dmc1 promotes ATP-dependent homologous DNA strand exchange. Nature 429(6990):433–437. doi:10.1038/nature02563
Ogawa T, Yu X, Shinohara A, Egelman EH (1993) Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259(5103):1896–1899
Galletto R, Amitani I, Baskin RJ, Kowalczykowski SC (2006) Direct observation of individual RecA filaments assembling on single DNA molecules. Nature 443(7113):875–878. doi:10.1038/nature05197
Handa N, Amitani I, Gumlaw N, Sandler SJ, Kowalczykowski SC (2009) Single molecule analysis of a red fluorescent RecA protein reveals a defect in nucleoprotein filament nucleation that relates to its reduced biological functions. J Biol Chem 284(28):18664–18673. doi:10.1074/jbc.M109.004895
Shin DS, Pellegrini L, Daniels DS, Yelent B, Craig L, Bates D, Yu DS, Shivji MK, Hitomi C, Arvai AS, Volkmann N, Tsuruta H, Blundell TL, Venkitaraman AR, Tainer JA (2003) Full-length archaeal Rad51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J 22(17):4566–4576. doi:10.1093/emboj/cdg429
Benson FE, Stasiak A, West SC (1994) Purification and characterization of the human Rad51 protein, an analogue of E. coli RecA. EMBO J 13(23):5764–5771
Sung P, Robberson DL (1995) DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82(3):453–461 (pii: 0092-8674(95)90434-4)
Gupta RC, Folta-Stogniew E, O’Malley S, Takahashi M, Radding CM (1999) Rapid exchange of A: T base pairs is essential for recognition of DNA homology by human Rad51 recombination protein. Mol Cell 4(5):705–714 (pii: S1097-2765(00)80381-0)
Yu X, Jacobs SA, West SC, Ogawa T, Egelman EH (2001) Domain structure and dynamics in the helical filaments formed by RecA and Rad51 on DNA. Proc Natl Acad Sci U S A 98(15):8419–8424. doi:10.1073/pnas.11100539898/15/8419
Stauffer ME, Chazin WJ (2004) Physical interaction between replication protein A and Rad51 promotes exchange on single-stranded DNA. J Biol Chem 279(24):25638–25645. doi:10.1074/jbc.M400029200
Fanning E, Klimovich V, Nager AR (2006) A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res 34(15):4126–4137. doi:10.1093/nar/gkl550
Fan J, Pavletich NP (2012) Structure and conformational change of a replication protein A heterotrimer bound to ssDNA. Genes Dev 26(20):2337–2347. doi:10.1101/gad.194787.112
Sigurdsson S, Trujillo K, Song B, Stratton S, Sung P (2001) Basis for avid homologous DNA strand exchange by human Rad51 and RPA. J Biol Chem 276(12):8798–8806. doi:10.1074/jbc.M010011200
Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, Blasco MA, Jonkers J, Tarsounas M (2010) BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol 17(12):1461–1469. doi:10.1038/nsmb.1943nsmb.1943
Flott S, Kwon Y, Pigli YZ, Rice PA, Sung P, Jackson SP (2011) Regulation of Rad51 function by phosphorylation. EMBO Rep 12(8):833–839. doi:10.1038/embor.2011.127
Krejci L, Altmannova V, Spirek M, Zhao X (2012) Homologous recombination and its regulation. Nucleic Acids Res 40(13):5795–5818. doi:10.1093/nar/gks270
Branzei D, Sollier J, Liberi G, Zhao X, Maeda D, Seki M, Enomoto T, Ohta K, Foiani M (2006) Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127(3):509–522. doi:10.1016/j.cell.2006.08.050
Yata K, Lloyd J, Maslen S, Bleuyard JY, Skehel M, Smerdon SJ, Esashi F (2012) Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell 45(3):371–383. doi:10.1016/j.molcel.2011.12.028
Sharan SK, Morimatsu M, Albrecht U, Lim DS, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A (1997) Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 386(6627):804–810. doi:10.1038/386804a0
Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC (2001) Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 7(2):273–282 (pii: S1097-2765(01)00175-7)
Levy-Lahad E, Lahad A, Eisenberg S, Dagan E, Paperna T, Kasinetz L, Catane R, Kaufman B, Beller U, Renbaum P, Gershoni-Baruch R (2001) A single nucleotide polymorphism in the RAD51 gene modifies cancer risk in BRCA2 but not BRCA1 carriers. Proc Natl Acad Sci USA 98(6):3232–3236. doi:10.1073/pnas.051624098
Xia F, Taghian DG, DeFrank JS, Zeng ZC, Willers H, Iliakis G, Powell SN (2001) Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc Natl Acad Sci USA 98(15):8644–8649. doi:10.1073/pnas.151253498
Lord CJ, Ashworth A (2007) RAD51, BRCA2 and DNA repair: a partial resolution. Nat Struct Mol Biol 14(6):461–462. doi:10.1038/nsmb0607-461
Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL (1997) RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. J Biol Chem 272(51):31941–31944
Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, Venkitaraman AR (2002) Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature 420(6913):287–293. doi:10.1038/nature01230
Shivji MK, Mukund SR, Rajendra E, Chen S, Short JM, Savill J, Klenerman D, Venkitaraman AR (2009) The BRC repeats of human BRCA2 differentially regulate RAD51 binding on single- versus double-stranded DNA to stimulate strand exchange. Proc Natl Acad Sci USA 106(32):13254–13259. doi:10.1073/pnas.0906208106
Carreira A, Hilario J, Amitani I, Baskin RJ, Shivji MK, Venkitaraman AR, Kowalczykowski SC (2009) The BRC repeats of BRCA2 modulate the DNA-binding selectivity of RAD51. Cell 136(6):1032–1043. doi:10.1016/j.cell.2009.02.019
Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, Couch FJ, Weber BL, Ashley T, Livingston DM, Scully R (1998) Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell 2(3):317–328 (pii: S1097-2765(00)80276-2)
Marmorstein LY, Ouchi T, Aaronson SA (1998) The BRCA2 gene product functionally interacts with p53 and RAD51. Proc Natl Acad Sci USA 95(23):13869–13874
Moynahan ME, Pierce AJ, Jasin M (2001) BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 7(2):263–272 (pii: S1097-2765(01)00174-5)
Kojic M, Kostrub CF, Buchman AR, Holloman WK (2002) BRCA2 homolog required for proficiency in DNA repair, recombination, and genome stability in Ustilago maydis. Mol Cell 10(3):683–691 (pii: S1097276502006329)
Yang H, Li Q, Fan J, Holloman WK, Pavletich NP (2005) The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction. Nature 433(7026):653–657. doi:10.1038/nature03234
Petalcorin MI, Galkin VE, Yu X, Egelman EH, Boulton SJ (2007) Stabilization of RAD-51-DNA filaments via an interaction domain in Caenorhabditis elegans BRCA2. Proc Natl Acad Sci U S A 104(20):8299–8304. doi:10.1073/pnas.0702805104
Jensen RB, Carreira A, Kowalczykowski SC (2010) Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467(7316):678–683. doi:10.1038/nature09399
Thorslund T, McIlwraith MJ, Compton SA, Lekomtsev S, Petronczki M, Griffith JD, West SC (2010) The breast cancer tumor suppressor BRCA2 promotes the specific targeting of RAD51 to single-stranded DNA. Nat Struct Mol Biol 17(10):1263–1265. doi:10.1038/nsmb.1905
Liu J, Doty T, Gibson B, Heyer WD (2010) Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol 17(10):1260–1262. doi:10.1038/nsmb.1904
Roy R, Chun J, Powell SN (2012) BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 12(1):68–78. doi:10.1038/nrc3181
Yu DS, Sonoda E, Takeda S, Huang CL, Pellegrini L, Blundell TL, Venkitaraman AR (2003) Dynamic control of Rad51 recombinase by self-association and interaction with BRCA2. Mol Cell 12(4):1029–1041 (pii: S1097276503003940)
Jensen RB, Ozes A, Kim T, Estep A, Kowalczykowski SC (2013) BRCA2 is epistatic to the RAD51 paralogs in response to DNA damage. DNA Repair (Amst) 12(4):306–311. doi:10.1016/j.dnarep.2012.12.007
Suwaki N, Klare K, Tarsounas M (2011) RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol 22(8):898–905. doi:10.1016/j.semcdb.2011.07.019
Thacker J (2005) The RAD51 gene family, genetic instability and cancer. Cancer Lett 219(2):125–135. doi:10.1016/j.canlet.2004.08.018
Liu N, Lamerdin JE, Tebbs RS, Schild D, Tucker JD, Shen MR, Brookman KW, Siciliano MJ, Walter CA, Fan W, Narayana LS, Zhou ZQ, Adamson AW, Sorensen KJ, Chen DJ, Jones NJ, Thompson LH (1998) XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol Cell 1(6):783–793 (pii: S1097-2765(00)80078-7)
Masson JY, Tarsounas MC, Stasiak AZ, Stasiak A, Shah R, McIlwraith MJ, Benson FE, West SC (2001) Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev 15(24):3296–3307. doi:10.1101/gad.947001
Takata M, Sasaki MS, Tachiiri S, Fukushima T, Sonoda E, Schild D, Thompson LH, Takeda S (2001) Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol Cell Biol 21(8):2858–2866. doi:10.1128/MCB.21.8.2858-2866.2001
French CA, Tambini CE, Thacker J (2003) Identification of functional domains in the RAD51L2 (RAD51C) protein and its requirement for gene conversion. J Biol Chem 278(46):45445–45450. doi:10.1074/jbc.M308621200
Hays SL, Firmenich AA, Berg P (1995) Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc Natl Acad Sci U S A 92(15):6925–6929
Sung P (1997) Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev 11(9):1111–1121
Huang J, Huen MS, Kim H, Leung CC, Glover JN, Yu X, Chen J (2009) RAD18 transmits DNA damage signalling to elicit homologous recombination repair. Nat Cell Biol 11(5):592–603. doi:10.1038/ncb1865ncb1865
Ting L, Jun H, Junjie C (2010) RAD18 lives a double life: Its implication in DNA double-strand break repair. DNA Repair (Amst) 9(12):1241–1248. doi:10.1016/j.dnarep.2010.09.016
Brenneman MA, Wagener BM, Miller CA, Allen C, Nickoloff JA (2002) XRCC3 controls the fidelity of homologous recombination: roles for XRCC3 in late stages of recombination. Mol Cell 10(2):387–395 (pii: S1097276502005956)
Liu Y, Masson JY, Shah R, O’Regan P, West SC (2004) RAD51C is required for Holliday junction processing in mammalian cells. Science 303(5655):243–246. doi:10.1126/science.1093037
Yoshihara T, Ishida M, Kinomura A, Katsura M, Tsuruga T, Tashiro S, Asahara T, Miyagawa K (2004) XRCC3 deficiency results in a defect in recombination and increased endoreduplication in human cells. EMBO J 23(3):670–680. doi:10.1038/sj.emboj.7600087
Liu T, Wan L, Wu Y, Chen J, Huang J (2011) hSWS1.SWSAP1 is an evolutionarily conserved complex required for efficient homologous recombination repair. J Biol Chem 286(48):41758–41766. doi:10.1074/jbc.M111.271080
Martin V, Chahwan C, Gao H, Blais V, Wohlschlegel J, Yates JR 3rd, McGowan CH, Russell P (2006) Sws1 is a conserved regulator of homologous recombination in eukaryotic cells. EMBO J 25(11):2564–2574. doi:10.1038/sj.emboj.7601141
Shor E, Weinstein J, Rothstein R (2005) A genetic screen for top3 suppressors in Saccharomyces cerevisiae identifies SHU1, SHU2, PSY3 and CSM2: four genes involved in error-free DNA repair. Genetics 169(3):1275–1289. doi:10.1534/genetics.104.036764
Mankouri HW, Ngo HP, Hickson ID (2007) Shu proteins promote the formation of homologous recombination intermediates that are processed by Sgs1-Rmi1-Top3. Mol Biol Cell 18(10):4062–4073. doi:10.1091/mbc.E07-05-0490
Ball LG, Zhang K, Cobb JA, Boone C, Xiao W (2009) The yeast Shu complex couples error-free post-replication repair to homologous recombination. Mol Microbiol 73(1):89–102. doi:10.1111/j.1365-2958.2009.06748.x
Bernstein KA, Reid RJ, Sunjevaric I, Demuth K, Burgess RC, Rothstein R (2011) The Shu complex, which contains Rad51 paralogues, promotes DNA repair through inhibition of the Srs2 anti-recombinase. Mol Biol Cell 22(9):1599–1607. doi:10.1091/mbc.E10-08-0691
Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM (2006) Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell 22(6):719–729. doi:10.1016/j.molcel.2006.05.022
Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T, Jayatilake H, McGuffog L, Hanks S, Evans DG, Eccles D, Easton DF, Stratton MR (2007) PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 39(2):165–167. doi:10.1038/ng1959
Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39(2):162–164. doi:10.1038/ng1947
Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, Wang W, Livingston DM, Joenje H, de Winter JP (2007) Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet 39(2):159–161. doi:10.1038/ng1942
Tischkowitz M, Xia B, Sabbaghian N, Reis-Filho JS, Hamel N, Li G, van Beers EH, Li L, Khalil T, Quenneville LA, Omeroglu A, Poll A, Lepage P, Wong N, Nederlof PM, Ashworth A, Tonin PN, Narod SA, Livingston DM, Foulkes WD (2007) Analysis of PALB2/FANCN-associated breast cancer families. Proc Natl Acad Sci USA 104(16):6788–6793. doi:10.1073/pnas.0701724104
Erkko H, Xia B, Nikkila J, Schleutker J, Syrjakoski K, Mannermaa A, Kallioniemi A, Pylkas K, Karppinen SM, Rapakko K, Miron A, Sheng Q, Li G, Mattila H, Bell DW, Haber DA, Grip M, Reiman M, Jukkola-Vuorinen A, Mustonen A, Kere J, Aaltonen LA, Kosma VM, Kataja V, Soini Y, Drapkin RI, Livingston DM, Winqvist R (2007) A recurrent mutation in PALB2 in Finnish cancer families. Nature 446(7133):316–319. doi:10.1038/nature05609
Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP (2009) Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324(5924):217. doi:10.1126/science.1171202
Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, Yu X (2009) PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol 19(6):524–529. doi:10.1016/j.cub.2009.02.018
Sy SM, Huen MS, Chen J (2009) PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A 106(17):7155–7160. doi:10.1073/pnas.0811159106
Dray E, Etchin J, Wiese C, Saro D, Williams GJ, Hammel M, Yu X, Galkin VE, Liu D, Tsai MS, Sy SM, Schild D, Egelman E, Chen J, Sung P (2010) Enhancement of RAD51 recombinase activity by the tumor suppressor PALB2. Nat Struct Mol Biol 17(10):1255–1259. doi:10.1038/nsmb.1916
Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP (2002) BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 297(5588):1837–1848. doi:10.1126/science.297.5588.1837
Kristensen CN, Bystol KM, Li B, Serrano L, Brenneman MA (2010) Depletion of DSS1 protein disables homologous recombinational repair in human cells. Mutat Res 694(1–2):60–64. doi:10.1016/j.mrfmmm.2010.08.007
Kojic M, Yang H, Kostrub CF, Pavletich NP, Holloman WK (2003) The BRCA2-interacting protein DSS1 is vital for DNA repair, recombination, and genome stability in Ustilago maydis. Mol Cell 12(4):1043–1049
Li J, Zou C, Bai Y, Wazer DE, Band V, Gao Q (2006) DSS1 is required for the stability of BRCA2. Oncogene 25(8):1186–1194. doi:10.1038/sj.onc.1209153
Zhou Q, Mazloum N, Mao N, Kojic M, Holloman WK (2009) Dss1 regulates interaction of Brh2 with DNA. Biochemistry 48(50):11929–11938. doi:10.1021/bi901775j
Kojic M, Zhou Q, Lisby M, Holloman WK (2005) Brh2-Dss1 interplay enables properly controlled recombination in Ustilago maydis. Mol Cell Biol 25(7):2547–2557. doi:10.1128/MCB.25.7.2547-2557.2005
Plank JL, Wu J, Hsieh TS (2006) Topoisomerase IIIalpha and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Natl Acad Sci USA 103(30):11118–11123. doi:10.1073/pnas.0604873103
Bzymek M, Thayer NH, Oh SD, Kleckner N, Hunter N (2010) Double Holliday junctions are intermediates of DNA break repair. Nature 464(7290):937–941. doi:10.1038/nature08868
Manthei KA, Keck JL (2013) The BLM dissolvasome in DNA replication and repair. Cell Mol Life Sci 70(21):4067–4084. doi:10.1007/s00018-013-1325-1
Larsen NB, Hickson ID (2013) RecQ Helicases: conserved guardians of genomic integrity. Adv Exp Med Biol 767:161–184. doi:10.1007/978-1-4614-5037-5-8
Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, West SC (2008) Identification of Holliday junction resolvases from humans and yeast. Nature 456(7220):357–361. doi:10.1038/nature07470
Svendsen JM, Harper JW (2010) GEN1/Yen1 and the SLX4 complex: solutions to the problem of Holliday junction resolution. Genes Dev 24(6):521–536. doi:10.1101/gad.1903510
Schwartz EK, Heyer WD (2011) Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma 120(2):109–127. doi:10.1007/s00412-010-0304-7
Boddy MN, Gaillard PH, McDonald WH, Shanahan P, Yates JR 3rd, Russell P (2001) Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107(4):537–548
Chen XB, Melchionna R, Denis CM, Gaillard PH, Blasina A, Van de Weyer I, Boddy MN, Russell P, Vialard J, McGowan CH (2001) Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell 8(5):1117–1127 (pii: S1097-2765(01)00375-6)
Constantinou A, Chen XB, McGowan CH, West SC (2002) Holliday junction resolution in human cells: two junction endonucleases with distinct substrate specificities. EMBO J 21(20):5577–5585
Gaillard PH, Noguchi E, Shanahan P, Russell P (2003) The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell 12(3):747–759 (pii: S1097276503003423)
Osman F, Dixon J, Doe CL, Whitby MC (2003) Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol Cell 12(3):761–774 (pii: S1097276503003435)
Taylor ER, McGowan CH (2008) Cleavage mechanism of human Mus81-Eme1 acting on Holliday-junction structures. Proc Natl Acad Sci U S A 105(10):3757–3762. doi:10.1073/pnas.0710291105
Svendsen JM, Smogorzewska A, Sowa ME, O’Connell BC, Gygi SP, Elledge SJ, Harper JW (2009) Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell 138(1):63–77. doi:10.1016/j.cell.2009.06.030
Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, Dong MQ, Ruse C, Yates JR 3rd, Russell P, Fuchs RP, McGowan CH, Gaillard PH (2009) Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138(1):78–89. doi:10.1016/j.cell.2009.06.029
Rass U, Compton SA, Matos J, Singleton MR, Ip SC, Blanco MG, Griffith JD, West SC (2010) Mechanism of Holliday junction resolution by the human GEN1 protein. Genes Dev 24(14):1559–1569. doi:10.1101/gad.58531024
Dehe PM, Coulon S, Scaglione S, Shanahan P, Takedachi A, Wohlschlegel JA, Yates JR 3rd, Llorente B, Russell P, Gaillard PH (2013) Regulation of Mus81-Eme1 Holliday junction resolvase in response to DNA damage. Nat Struct Mol Biol 20(5):598–603. doi:10.1038/nsmb.2550
Wyatt HD, Sarbajna S, Matos J, West SC (2013) Coordinated actions of SLX1-SLX4 and MUS81-EME1 for Holliday junction resolution in human cells. Mol Cell 52(2):234–247. doi:10.1016/j.molcel.2013.08.035
Castor D, Declais AC, Lachaud C, Toth R, Macartney TJ, Lilley DM, Arthur JS, Rouse J (2013) Cooperative control of holliday junction resolution and DNA repair by the SLX1 and MUS81-EME1 nucleases. Mol Cell 52(2):221–233. doi:10.1016/j.molcel.2013.08.036
Wilson JS, Tejera AM, Castor D, Toth R, Blasco MA, Rouse J (2013) Localization-dependent and -independent roles of SLX4 in regulating telomeres. Cell Rep 4(5):853–860. doi:10.1016/j.celrep.2013.07.033
Wan B, Yin J, Horvath K, Sarkar J, Chen Y, Wu J, Wan K, Lu J, Gu P, Yu EY, Lue NF, Chang S, Liu Y, Lei M (2013) SLX4 assembles a telomere maintenance toolkit by bridging multiple endonucleases with telomeres. Cell Rep 4(5):861–869. doi:10.1016/j.celrep.2013.08.017
Karow JK, Chakraverty RK, Hickson ID (1997) The Bloom’s syndrome gene product is a 3′-5′ DNA helicase. J Biol Chem 272(49):30611–30614
Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J (1995) The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell 83(4):655–666 (pii: 0092-8674(95)90105-1)
Gruber SB, Ellis NA, Scott KK, Almog R, Kolachana P, Bonner JD, Kirchhoff T, Tomsho LP, Nafa K, Pierce H, Low M, Satagopan J, Rennert H, Huang H, Greenson JK, Groden J, Rapaport B, Shia J, Johnson S, Gregersen PK, Harris CC, Boyd J, Rennert G, Offit K (2002) BLM heterozygosity and the risk of colorectal cancer. Science 297(5589):2013. doi:10.1126/science.1074399
Goss KH, Risinger MA, Kordich JJ, Sanz MM, Straughen JE, Slovek LE, Capobianco AJ, German J, Boivin GP, Groden J (2002) Enhanced tumor formation in mice heterozygous for Blm mutation. Science 297(5589):2051–2053. doi:10.1126/science.1074340
Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W (2005) BLAP75, an essential component of Bloom’s syndrome protein complexes that maintain genome integrity. EMBO J 24(7):1465–1476. doi:10.1038/sj.emboj.7600622
Seki M, Nakagawa T, Seki T, Kato G, Tada S, Takahashi Y, Yoshimura A, Kobayashi T, Aoki A, Otsuki M, Habermann FA, Tanabe H, Ishii Y, Enomoto T (2006) Bloom helicase and DNA topoisomerase IIIalpha are involved in the dissolution of sister chromatids. Mol Cell Biol 26(16):6299–6307. doi:10.1128/MCB.00702-06
Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W (2008) RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev 22(20):2843–2855. doi:10.1101/gad.1708608
Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, Andreassen PR, Sung P, Meetei AR (2008) BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev 22(20):2856–2868. doi:10.1101/gad.1725108
Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R (1989) A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58(2):409–419 (pii: 0092-8674(89)90855-6)
Hanai R, Caron PR, Wang JC (1996) Human TOP3: a single-copy gene encoding DNA topoisomerase III. Proc Natl Acad Sci USA 93(8):3653–3657
Hu P, Beresten SF, van Brabant AJ, Ye TZ, Pandolfi PP, Johnson FB, Guarente L, Ellis NA (2001) Evidence for BLM and Topoisomerase IIIalpha interaction in genomic stability. Hum Mol Genet 10(12):1287–1298
Plank J, Hsieh TS (2009) Helicase-appended topoisomerases: new insight into the mechanism of directional strand transfer. J Biol Chem 284(45):30737–30741. doi:10.1074/jbc.R109.051268
Raynard S, Zhao W, Bussen W, Lu L, Ding YY, Busygina V, Meetei AR, Sung P (2008) Functional role of BLAP75 in BLM-topoisomerase IIIalpha-dependent holliday junction processing. J Biol Chem 283(23):15701–15708. doi:10.1074/jbc.M802127200
Raynard S, Bussen W, Sung P (2006) A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J Biol Chem 281(20):13861–13864. doi:10.1074/jbc.C600051200
Franchitto A, Pichierri P (2002) Bloom’s syndrome protein is required for correct relocalization of RAD50/MRE11/NBS1 complex after replication fork arrest. J Cell Biol 157(1):19–30. doi:10.1083/jcb.200110009
Bugreev DV, Yu X, Egelman EH, Mazin AV (2007) Novel pro- and anti-recombination activities of the Bloom’s syndrome helicase. Genes Dev 21(23):3085–3094. doi:10.1101/gad.1609007
Gravel S, Chapman JR, Magill C, Jackson SP (2008) DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev 22(20):2767–2772. doi:10.1101/gad.503108
Chu WK, Hanada K, Kanaar R, Hickson ID (2010) BLM has early and late functions in homologous recombination repair in mouse embryonic stem cells. Oncogene 29(33):4705–4714. doi:10.1038/onc.2010.214
Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC (2011) BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev 25(4):350–362. doi:10.1101/gad.2003811
Wan L, Han J, Liu T, Dong S, Xie F, Chen H, Huang J (2013) Scaffolding protein SPIDR/KIAA0146 connects the Bloom syndrome helicase with homologous recombination repair. Proc Natl Acad Sci USA 110(26):10646–10651. doi:10.1073/pnas.1220921110
Bischof O, Kim SH, Irving J, Beresten S, Ellis NA, Campisi J (2001) Regulation and localization of the Bloom syndrome protein in response to DNA damage. J Cell Biol 153(2):367–380
Tutt A, Bertwistle D, Valentine J, Gabriel A, Swift S, Ross G, Griffin C, Thacker J, Ashworth A (2001) Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J 20(17):4704–4716. doi:10.1093/emboj/20.17.4704
Feng Z, Zhang J (2012) A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest. Nucleic Acids Res 40(2):726–738. doi:10.1093/nar/gkr748
Yuan J, Chen J (2013) FIGNL1-containing protein complex is required for efficient homologous recombination repair. Proc Natl Acad Sci USA 110(26):10640–10645. doi:10.1073/pnas.1220662110
Acknowledgments
We apologize to colleagues whose work could not be cited due to space limitations. We would like to thank all our colleagues in the Huang laboratory for insightful discussions. This work was supported in part by National Program for Special Support of Eminent Professionals, National Basic Research Program of China Grants 2012CB944402 and 2013CB911003, National Natural Science Funds for Distinguished Young Scholar, National Natural Science Foundation of China Grant 31071243, Zhejiang University K.P. Chao’s High Technology Development Foundation, and the China’s Fundamental Research Funds for the Central Universities. Ting Liu is a member of Feng lab and supported by National Natural Science Foundation of China grant (31171347 and 31090360) and MOST Grants 2012CB966600 and 2013CB945303.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, T., Huang, J. Quality control of homologous recombination. Cell. Mol. Life Sci. 71, 3779–3797 (2014). https://doi.org/10.1007/s00018-014-1649-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1649-5