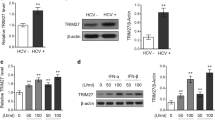
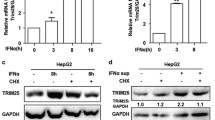
Abstract
Hepatitis C virus (HCV) infection is associated with hepatic iron overload and elevated serum iron that correlate to poor antiviral responses. Hepcidin (HAMP), a 25-aa cysteine-rich liver-specific peptide, controls iron homeostasis. Its expression is up-regulated in inflammation and iron excess. HCV-mediated hepcidin regulation remains controversial. Chronic HCV patients possess relatively low hepcidin levels; however, elevated HAMP mRNA has been reported in HCV core transgenic mice and HCV replicon-expressing cells. We investigated the effect of HCV core protein on HAMP gene expression and delineated the complex interplay of molecular mechanisms involved. HCV core protein up-regulated HAMP promoter activity, mRNA, and secreted protein levels. Enhanced promoter activity was abolished by co-transfections of core with HAMP promoter constructs containing mutated/deleted BMP and STAT binding sites. Dominant negative constructs, pharmacological inhibitors, and silencing experiments against STAT3 and SMAD4 confirmed the participation of both pathways in HAMP gene regulation by core protein. STAT3 and SMAD4 expression levels were found increased in the presence of HCV core, which orchestrated SMAD4 translocation into the nucleus and STAT3 phosphorylation. To further understand the mechanisms governing the core effect, the role of the JAK/STAT-activating kinase CK2 was investigated. A CK2-dominant negative construct, a CK2-specific inhibitor, and RNAi interference abrogated the core-induced increase on HAMP promoter activity, mRNA, and protein levels, while CK2 acted in synergy with core to significantly enhance HAMP gene expression. Therefore, HCV core up-regulates HAMP gene transcription via a complex signaling network that requires both SMAD/BMP and STAT3 pathways and CK2 involvement.






Similar content being viewed by others
References
Valore EV, Ganz T (2008) Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis 40(1):132–138
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113(9):1271–1276
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1(3):191–200
Camaschella C, Poggiali E (2011) Inherited disorders of iron metabolism. Curr Opin Pediatr 23(1):14–20
Finberg KE (2013) Regulation of systemic iron homeostasis. Curr Opin Hematol 20(3):208–214
Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101(7):2461–2463
Pietrangelo A (2011) Hepcidin in human iron disorders: therapeutic implications. J Hepatol 54(1):173–181
Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY (2006) Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38(5):531–539
Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU (2007) STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109(1):353–358
Bayele HK, McArdle H, Srai SK (2006) Cis and trans regulation of hepcidin expression by upstream stimulatory factor. Blood 108(13):4237–4245
Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU (2009) Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 87(5):471–480. doi:10.1007/s00109-009-0447-2
Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P, Loreal O, Ilyin G (2002) C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem 277(43):41163–41170 Epub 42002 Aug 41114
Island ML, Fatih N, Leroyer P, Brissot P, Loreal O (2011) GATA-4 transcription factor regulates hepatic hepcidin expression. Biochem J 437(3):477–482
Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, Dooley S, Hentze MW, Muckenthaler MU (2010) SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood 115(13):2657–2665
Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS (2007) Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 117(7):1926–1932
Truksa J, Lee P, Beutler E (2007) The role of STAT, AP-1, E-box and TIEG motifs in the regulation of hepcidin by IL-6 and BMP-9: lessons from human HAMP and murine Hamp1 and Hamp2 gene promoters. Blood Cells Mol Dis 39(3):255–262
Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, Rechavi G (2007) Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol 138(2):253–262
Wu S, Zhang K, Lv C, Wang H, Cheng B, Jin Y, Chen Q, Lian Q, Fang X (2012) Nuclear factor-kappaB mediated lipopolysaccharide-induced mRNA expression of hepcidin in human peripheral blood leukocytes. Innate Immun 18(2):318–324
Poenisch M, Bartenschlager R (2010) New insights into structure and replication of the hepatitis C virus and clinical implications. Semin Liver Dis 30(4):333–347
Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G (2005) Review article: hepatitis C virus-associated steatosis–pathogenic mechanisms and clinical implications. Aliment Pharmacol Ther 22(Suppl 2):52–55
Lonardo A, Loria P, Carulli N (2008) Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med 3(2):99–108. doi:10.1007/s11739-008-0127-1
Metwally MA, Zein CO, Zein NN (2004) Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol 99(2):286–291
Isom HC, McDevitt EI, Moon MS (2009) Elevated hepatic iron: a confounding factor in chronic hepatitis C. Biochim Biophys Acta 1790(7):650–662. doi:10.1016/j.bbagen.2009.04.009
Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M (2007) Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med 13(1–2):97–104
Girelli D, Pasino M, Goodnough JB, Nemeth E, Guido M, Castagna A, Busti F, Campostrini N, Martinelli N, Vantini I, Corrocher R, Ganz T, Fattovich G (2009) Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol 51(5):845–852
Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN, Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG (2013) HCV and oxidative stress in the liver hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. Viruses 5(2):439–469
Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ (2010) Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat 17(11):800–806
Polyak SJ, Klein KC, Shoji I, Miyamura T, Lingappa JR (2006) Assemble and Interact: Pleiotropic Functions of the HCV Core Protein. In: Tan SL (ed) Source Hepatitis C Viruses: Genomes and Molecular Biology, Chap 3. Norfolk, Horizon Bioscience
Koike K (2009) Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol 44(Suppl 19):82–88. doi:10.1007/s00535-00008-02276-00534
McLauchlan J (2000) Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J Viral Hepat 7(1):2–14
Ait-Goughoulte M, Banerjee A, Meyer K, Mazumdar B, Saito K, Ray RB, Ray R (2010) Hepatitis C virus core protein interacts with fibrinogen-beta and attenuates cytokine stimulated acute-phase response. Hepatology 51(5):1505–1513
Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F (2012) Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol 91(6–7):496–505
Moshage H (1997) Cytokines and the hepatic acute phase response. J Pathol 181(3):257–266
Johnson EE, Wessling-Resnick M (2012) Iron metabolism and the innate immune response to infection. Microbes Infect 14(3):207–216
Lee P, Peng H, Gelbart T, Wang L, Beutler E (2005) Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA 102(6):1906–1910
Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H (2011) Hepcidin regulation by innate immune and infectious stimuli. Blood 118(15):4129–4139
Fleming RE (2007) Hepcidin activation during inflammation: make it STAT. Gastroenterology 132(1):447–449
Park SO, Kumar M, Gupta S (2012) TGF-beta and iron differently alter HBV replication in human hepatocytes through TGF-beta/BMP signaling and cellular microRNA expression. PLoS One 7(6):18
Truksa J, Lee P, Beutler E (2009) Two BMP responsive elements, STAT, and bZIP/HNF4/COUP motifs of the hepcidin promoter are critical for BMP, SMAD1, and HJV responsiveness. Blood 113(3):688–695
Braliou GG, Verga Falzacappa MV, Chachami G, Casanovas G, Muckenthaler MU, Simos G (2008) 2-Oxoglutarate-dependent oxygenases control hepcidin gene expression. J Hepatol 48(5):801–810. doi:10.1016/j.jhep.2007.12.021
Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE Jr (1998) Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol 18(5):2553–2558
Zhang Y, Feng XH, Derynck R (1998) Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 394(6696):909–913
Heriche JK, Lebrin F, Rabilloud T, Leroy D, Chambaz EM, Goldberg Y (1997) Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science 276(5314):952–955
Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA (2006) Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett 580(16):3948–3952
Katsarou K, Tsitoura P, Georgopoulou U (2011) MEK5/ERK5/mef2: a novel signaling pathway affected by hepatitis C virus non-enveloped capsid-like particles. Biochim Biophys Acta 10:1854–1862
Foka P, Pourchet A, Hernandez-Alcoceba R, Doumba PP, Pissas G, Kouvatsis V, Dalagiorgou G, Kazazi D, Marconi P, Foschini M, Manservigi R, Konstadoulakis MM, Koskinas J, Epstein AL, Mavromara P (2010) Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J Gene Med 12(12):956–967
Keller C, Keller P, Marshal S, Pedersen BK (2003) IL-6 gene expression in human adipose tissue in response to exercise—effect of carbohydrate ingestion. J Physiol 550(Pt 3):927–931
Moriguchi M, Terai C, Koseki Y, Uesato M, Nakajima A, Inada S, Nishinarita M, Uchida S, Kim SY, Chen CL, Kamatani N (1999) Influence of genotypes at SAA1 and SAA2 loci on the development and the length of latent period of secondary AA-amyloidosis in patients with rheumatoid arthritis. Hum Genet 105(4):360–366
Tsitoura E, Thomas J, Cuchet D, Thoinet K, Mavromara P, Epstein AL (2009) Infection with herpes simplex type 1-based amplicon vectors results in an IRF3/7-dependent, TLR-independent activation of the innate antiviral response in primary human fibroblasts. J Gen Virol 90(Pt 9):2209–2220
Koliaraki V, Marinou M, Samiotaki M, Panayotou G, Pantopoulos K, Mamalaki A (2008) Iron regulatory and bactericidal properties of human recombinant hepcidin expressed in Pichia pastoris. Biochimie 90(5):726–735. doi:10.1016/j.biochi.2008.01.012
Katsarou K, Lavdas AA, Tsitoura P, Serti E, Markoulatos P, Mavromara P, Georgopoulou U (2010) Endocytosis of hepatitis C virus non-enveloped capsid-like particles induces MAPK-ERK1/2 signaling events. Cell Mol Life Sci 67(14):2491–2506
Tsitoura P, Georgopoulou U, Petres S, Varaklioti A, Karafoulidou A, Vagena D, Politis C, Mavromara P (2007) Evidence for cellular uptake of recombinant hepatitis C virus non-enveloped capsid-like particles. FEBS Lett 581(21):4049–4057
Ngo HT, Pham LV, Kim JW, Lim YS, Hwang SB (2013) Modulation of mitogen-activated protein kinase-activated protein kinase 3 by hepatitis C virus core protein. J Virol 87(10):5718–5731
Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O, Hassan M (2012) Hepatitis C virus-related hepatocellular carcinoma: an insight into molecular mechanisms and therapeutic strategies. World 4(12):342–355
Yan XB, Chen Z, Brechot C (2010) Associations among genotype 1b hepatitis C virus core protein, protein kinase R, and signal transducer and activator of transcription 3. Hepat Mon 10(4):275–284
Verga Falzacappa MV, Casanovas G, Hentze MW, Muckenthaler MU (2008) A bone morphogenetic protein (BMP)—responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med (Berl) 86(5):531–540. doi:10.1007/s00109-008-0313-7
Anderson GJ, Darshan D (2008) Small-molecule dissection of BMP signaling. Nat Chem Biol 4(1):15–16
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 16(17):5353–5362
Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K (2004) IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun 314(2):363–369
Bragdon B, Thinakaran S, Moseychuk O, King D, Young K, Litchfield DW, Petersen NO, Nohe A (2010) Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys J 99(3):897–904
Zheng Y, Qin H, Frank SJ, Deng L, Litchfield DW, Tefferi A, Pardanani A, Lin FT, Li J, Sha B, Benveniste EN (2011) A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood 118(1):156–166
Cozza G, Mazzorana M, Papinutto E, Bain J, Elliott M, di Maira G, Gianoncelli A, Pagano MA, Sarno S, Ruzzene M, Battistutta R, Meggio F, Moro S, Zagotto G, Pinna LA (2009) Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem J 421(3):387–395
Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K (2006) Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res 36(4):288–293
Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I, Okita K, Sakaida I (2008) Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology 134(1):226–238. doi:10.1053/j.gastro.2007.10.011
Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA (2008) Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology 48(5):1420–1429
Moriya K, Miyoshi H, Shinzawa S, Tsutsumi T, Fujie H, Goto K, Shintani Y, Yotsuyanagi H, Koike K (2010) Hepatitis C virus core protein compromises iron-induced activation of antioxidants in mice and HepG2 cells. J Med Virol 82(5):776–792
Miyachi H, Kobayashi Y, Relja B, Fujita N, Iwasa M, Gabazza EC, Takei Y (2011) Effect of suppressor of cytokine signaling on hepcidin production in hepatitis C virus replicon cells. Hepatol Res 41(4):364–374
Kochlios E, Foka P, Tsitoura E, Doumba PP, Koskinas J, Mavromara P (2010) Effect of HCV core and core +1/S on pro- and anti-inflammatory cytokine and chemokine gene expression. In: 8th Joint Conference of the International Cytokine Society and the International Society for Interferon and Cytokine Research Chicago (USA), October 3–7, 2010, pp 21–24
Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS (2011) Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J Biol Chem 286(12):10847–10855
Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD (2009) Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139(3):434–438. doi:10.3945/jn.108.094052
Kato J, Kobune M, Ohkubo S, Fujikawa K, Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y, Niitsu Y (2007) Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol 35(6):879–887
Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, Lopez-Otin C (2009) Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica 94(6):840–849. doi:10.3324/haematol.2008.001867
Zhang AS, Anderson SA, Wang J, Yang F, DeMaster K, Ahmed R, Nizzi CP, Eisenstein RS, Tsukamoto H, Enns CA (2011) Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood 117(5):1687–1699
Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL (2009) BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41(4):482–487. doi:10.1038/ng.335
Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL (2011) Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology 54(1):273–284
Truksa J, Peng H, Lee P, Beutler E (2006) Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA 103(27):10289–10293
Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX (2005) A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2(6):399–409
Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP (2008) Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 112(4):1503–1509. doi:10.1182/blood-2008-03-143354
Murata T, Ohshima T, Yamaji M, Hosaka M, Miyanari Y, Hijikata M, Shimotohno K (2005) Suppression of hepatitis C virus replicon by TGF-beta. Virology 331(2):407–417
Sakamoto N, Yoshimura M, Kimura T, Toyama K, Sekine-Osajima Y, Watanabe M, Muramatsu M (2007) Bone morphogenetic protein-7 and interferon-alpha synergistically suppress hepatitis C virus replicon. Biochem Biophys Res Commun 357(2):467–473
Pavio N, Battaglia S, Boucreux D, Arnulf B, Sobesky R, Hermine O, Brechot C (2005) Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene 24(40):6119–6132
Wrighting DM, Andrews NC (2006) Interleukin-6 induces hepcidin expression through STAT3. Blood 108(9):3204–3209
Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W (2000) Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J 347(Pt 1):89–96
Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C (2007) STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology 132(1):294–300
Pandur E, Sipos K, Grama L, Nagy J, Poor VS, Setalo G, Miseta A, Fekete Z (2013) Prohepcidin binds to the HAMP promoter and autoregulates its own expression. Biochem J 451(2):301–311
Choi SO, Cho YS, Kim HL, Park JW (2007) ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem Biophys Res Commun 356(1):312–317
Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110(7):1037–1044
Millonig G, Ganzleben I, Peccerella T, Casanovas G, Brodziak-Jarosz L, Breitkopf-Heinlein K, Dick TP, Seitz HK, Muckenthaler MU, Mueller S (2012) Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3). J Biol Chem 287(44):37472–37482
Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A (2002) Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med 196(5):641–653
Huang H, Constante M, Layoun A, Santos MM (2009) Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood 113(15):3593–3599. doi:10.1182/blood-2008-08-173641
Sun CC, Vaja V, Babitt JL, Lin HY (2012) Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol 87(4):392–400
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4(1):33–41
Sass G, Klinger N, Sirma H, Hashemolhosseini S, Hellerbrand C, Neureiter D, Wege H, Ocker M, Tiegs G (2011) Inhibition of experimental HCC growth in mice by use of the kinase inhibitor DMAT. Int J Oncol 39(2):433–44294
Ruzzene M, Pinna LA (2010) Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta 3:499–504
Bretana NA, Lu CT, Chiang CY, Su MG, Huang KY, Lee TY, Weng SL (2012) Identifying protein phosphorylation sites with kinase substrate specificity on human viruses. PLoS One 7(7):23
Kim J, Lee D, Choe J (1999) Hepatitis C virus NS5A protein is phosphorylated by casein kinase II. Biochem Biophys Res Commun 257(3):777–781
Lin YC, Hung MS, Lin CK, Li JM, Lee KD, Li YC, Chen MF, Chen JK, Yang CT (2011) CK2 inhibitors enhance the radiosensitivity of human non-small cell lung cancer cells through inhibition of stat3 activation. Cancer Biother Radiopharm 26(3):381–388
Piazza FA, Ruzzene M, Gurrieri C, Montini B, Bonanni L, Chioetto G, Di Maira G, Barbon F, Cabrelle A, Zambello R, Adami F, Trentin L, Pinna LA, Semenzato G (2006) Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood 108(5):1698–1707
Harvey EJ, Li N, Ramji DP (2007) Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-gamma-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis. Arterioscler Thromb Vasc Biol 27(4):806–812
Drygin D, Ho CB, Omori M, Bliesath J, Proffitt C, Rice R, Siddiqui-Jain A, O’Brien S, Padgett C, Lim JK, Anderes K, Rice WG, Ryckman D (2011) Protein kinase CK2 modulates IL-6 expression in inflammatory breast cancer. Biochem Biophys Res Commun 415(1):163–167
Chaverneff F, Barrett J (2009) Casein kinase II contributes to the synergistic effects of BMP7 and BDNF on Smad 1/5/8 phosphorylation in septal neurons under hypoglycemic stress. J Neurochem 109(3):733–743
Hu WT, Li HC, Lee SK, Ma HC, Yang CH, Chen HL, Lo SY (2013) Both core and F proteins of hepatitis C virus could enhance cell proliferation in transgenic mice. Biochem Biophys Res Commun 435(1):147–152
Acknowledgments
We are grateful to Dr. P. Lee (The Scripps Research Institute, USA), Prof. M.U. Muckenthaler (University of Heidelberg, Germany), Dr. J.E. Darnell (Rockefeller University, NY, USA), Dr. R. Derynck (University of California at San Francisco) and Dr. C. Cochet (INSERM U1036, Grenoble, France) for providing promoter constructs and expression plasmids used in this study. Financial support was provided in the context of project 09SYN-12-682, which is implemented under the auspices of NSRF and the National Range Action “COOPERATION” and co-funded by the Greek Government and the European Union - European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding authors
Additional information
P. Foka and A. Dimitriadis contributed equally to this work and should be regarded as joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foka, P., Dimitriadis, A., Kyratzopoulou, E. et al. A complex signaling network involving protein kinase CK2 is required for hepatitis C virus core protein-mediated modulation of the iron-regulatory hepcidin gene expression. Cell. Mol. Life Sci. 71, 4243–4258 (2014). https://doi.org/10.1007/s00018-014-1621-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1621-4