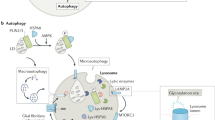
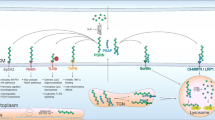
Abstract
The ubiquitous distribution of lysosomes and their heterogeneous protein composition reflects the versatility of these organelles in maintaining cell homeostasis and their importance in tissue differentiation and remodeling. In lysosomes, the degradation of complex, macromolecular substrates requires the synergistic action of multiple hydrolases that usually work in a stepwise fashion. This catalytic machinery explains the existence of lysosomal enzyme complexes that can be dynamically assembled and disassembled to efficiently and quickly adapt to the pool of substrates to be processed or degraded, adding extra tiers to the regulation of the individual protein components. An example of such a complex is the one composed of three hydrolases that are ubiquitously but differentially expressed: the serine carboxypeptidase, protective protein/cathepsin A (PPCA), the sialidase, neuraminidase-1 (NEU1), and the glycosidase β-galactosidase (β-GAL). Next to this ‘core’ complex, the existence of sub-complexes, which may contain additional components, and function at the cell surface or extracellularly, suggests as yet unexplored functions of these enzymes. Here we review how studies of basic biological processes in the mouse models of three lysosomal storage disorders, galactosialidosis, sialidosis, and GM1-gangliosidosis, revealed new and unexpected roles for the three respective affected enzymes, Ppca, Neu1, and β-Gal, that go beyond their canonical degradative activities. These findings have broadened our perspective on their functions and may pave the way for the development of new therapies for these lysosomal storage disorders.





Similar content being viewed by others
Abbreviations
- ASMC:
-
Aortic smooth muscle cell
- BiP:
-
Binding immunoglobulin protein
- β-GAL:
-
β-Galactosidase
- BM:
-
Bone marrow
- CHOP:
-
CCAAT/enhancer-binding protein homologous protein
- CMA:
-
Chaperone-mediated autophagy
- EBP:
-
Elastin-binding protein
- ECM:
-
Extracellular matrix
- EMH:
-
Extramedullary hematopoiesis
- ER:
-
Endoplasmic reticulum
- GALNS:
-
N-acetylgalactosamine-6-sulfate sulfatase
- GEMs:
-
Glysosphingolipid-enriched microdomains
- GM1:
-
GM1-gangliosidosis
- GPCR:
-
Guanine protein-coupled receptor
- GS:
-
Galactosialidosis
- Hsp:
-
Heat shock protein
- IGF-1R:
-
Insulin-like growth factor 1 receptor
- IP3R:
-
Inositol trisphosphate receptor
- JNK:
-
c-Jun N-terminal kinases
- LAMP:
-
Lysosomal membrane-associated protein
- LM:
-
Lysosomal membrane
- LMC:
-
Lysosomal multienzyme complex
- LPS:
-
Lipopolysaccharide
- LSD:
-
Lysosomal storage disorder
- MAMs:
-
Mitochondria-associated ER membranes
- MAPK/ERK:
-
Mitogen-activated protein kinase
- MAP2K/MEK:
-
Mitogen-activated protein kinase kinase
- MPP:
-
Metalloproteinase
- MyD88:
-
Myeloid differentiation primary response gene (88)
- NEU1:
-
Neuraminidase-1
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PDGF-R:
-
Platelet-derived growth factor receptor
- PI3K:
-
Phosphoinositide 3-kinase
- PLCγ:
-
Phospholipase C gamma
- PM:
-
Plasma membrane
- PPCA:
-
Protective protein/cathepsin A
- Ras:
-
Rapidly accelerated fibrosarcoma
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UPR:
-
Unfolded protein response
- VCAM1:
-
Vascular cell adhesion molecule 1
References
D’Azzo A, Hoogeveen A, Reuser AJ, Robinson D, Galjaard H (1982) Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci USA 79(15):4535–4539
Verheijen F, Brossmer R, Galjaard H (1982) Purification of acid beta-galactosidase and acid neuraminidase from bovine testis: evidence for an enzyme complex. Biochem Biophys Res Commun 108(2):868–875
Galjart NJ, Gillemans N, Harris A, van der Horst GT, Verheijen FW, Galjaard H, d’Azzo A (1988) Expression of cDNA encoding the human “protective protein” associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell 54(6):755–764
Bonten EJ, Galjart NJ, Willemsen R, Usmany M, Vlak JM, d’Azzo A (1995) Lysosomal protective protein/cathepsin A. Role of the “linker” domain in catalytic activation. J Biol Chem 270(44):26441–26445
Pshezhetsky AV, Potier M (1996) Association of N-acetylgalactosamine-6-sulfate sulfatase with the multienzyme lysosomal complex of beta-galactosidase, cathepsin A, and neuraminidase. Possible implication for intralysosomal catabolism of keratan sulfate. J Biol Chem 271(45):28359–28365
van der Spoel A, Bonten E, d’Azzo A (2000) Processing of lysosomal beta-galactosidase. The C-terminal precursor fragment is an essential domain of the mature enzyme. J Biol Chem 275(14):10035–10040
Bonten EJ, d’Azzo A (2000) Lysosomal neuraminidase. Catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J Biol Chem 275(48):37657–37663
van der Spoel A, Bonten E, d’Azzo A (1998) Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J 17(6):1588–1597
Galjart NJ, Gillemans N, Harris A, van der Horst GTJ, Verheijen FW, Galjaard H, d’Azzo A (1988) Expression of cDNA encoding the human protective protein associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell 54:755–764
Jackman HL, Morris PW, Deddish PA, Skidgel RA, Erdos EG (1992) Inactivation of endothelin I by deamidase (lysosomal protective protein). J Biol Chem 267(5):2872–2875
Hanna WL, Turbov JM, Jackman HL, Tan F, Froelich CJ (1994) Dominant chymotrypsin-like esterase activity in human lymphocyte granules is mediated by the serine carboxypeptidase called cathepsin A-like protective protein. J Immunol 153(10):4663–4672
Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdos EG (2002) Angiotensin 1–9 and 1–7 release in human heart: role of cathepsin A. Hypertension 39(5):976–981
Rudenko G, Bonten E, d’Azzo A, Hol WG (1996) Structure determination of the human protective protein: twofold averaging reveals the three-dimensional structure of a domain which was entirely absent in the initial model. Acta Crystallogr D Biol Crystallogr 52(Pt 5):923–936
Rudenko G, Bonten E, d’Azzo A, Hol WG (1995) Three-dimensional structure of the human ‘protective protein’: structure of the precursor form suggests a complex activation mechanism. Structure 3(11):1249–1259
Bonten EJ, Campos Y, Zaitsev V, Nourse A, Waddell B, Lewis W, Taylor G, d’Azzo A (2009) Heterodimerization of the sialidase NEU1 with the chaperone protective protein/cathepsin A prevents its premature oligomerization. J Biol Chem 284(41):28430–28441
Galjart NJ, Morreau H, Willemsen R, Gillemans N, Bonten EJ, d’Azzo A (1991) Human lysosomal protective protein has cathepsin A-like activity distinct from its protective function. J Biol Chem 266(22):14754–14762
d’Azzo A, Andria G, Strisciuglio P, Galjaard H (2001) Galactosialidosis. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited disease, vol 3, 8th edn. McGraw-Hill Publishing Co., New York, pp 3811–3826
Thomas GH (2001) Disorders of glycoprotein degradation and structure: α-mannosidosis, β-mannosidosis, fucosidosis, and sialidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol III, 8th edn. McGraw Hill Inc., New York, pp 3507–3534
Suzuki YAO, Namba E (2001) β-Galactosidase deficiency (β-Galactosialidosis): GM1 gangliosidosis and morquio B disease. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill Publishing Co, New York, pp 3775–3810
Caciotti A, Garman SC, Rivera-Colon Y, Procopio E, Catarzi S, Ferri L, Guido C, Martelli P, Parini R, Antuzzi D, Battini R, Sibilio M, Simonati A, Fontana E, Salviati A, Akinci G, Cereda C, Dionisi-Vici C, Deodato F, d’Amico A, d’Azzo A, Bertini E, Filocamo M, Scarpa M, di Rocco M, Tifft CJ, Ciani F, Gasperini S, Pasquini E, Guerrini R, Donati MA, Morrone A (2011) GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochim Biophys Acta 1812(7):782–790
Caciotti A, Catarzi S, Tonin R, Lugli L, Perez CR, Michelakakis H, Mavridou I, Donati MA, Guerrini R, d’Azzo A, Morrone A (2013) Galactosialidosis: review and analysis of CTSA gene mutations. Orphanet J Rare Dis 8:114
d’Azzo A, Bonten E (2010) Molecular mechanisms of pathogenesis in a glycosphingolipid and a glycoprotein storage disease. Biochem Soc Trans 38(6):1453–1457
de Geest N, Bonten E, Mann L, de Sousa-Hitzler J, Hahn C, d’Azzo A (2002) Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum Mol Genet 11(12):1455–1464
Zhou XY, Morreau H, Rottier R, Davis D, Bonten E, Gillemans N, Wenger D, Grosveld FG, Doherty P, Suzuki K, Grosveld GC, d’Azzo A (1995) Mouse model for the lysosomal disorder galactosialidosis and correction of the phenotype with over-expressing erythroid precursor cells. Genes Dev 9:2623–2634
Rottier RJ, Hahn CN, Mann LW, del Pilar Martin M, Smeyne RJ, Suzuki K, d’Azzo A (1998) Lack of PPCA expression only partially coincides with lysosomal storage in galactosialidosis mice: indirect evidence for spatial requirement of the catalytic rather than the protective function of PPCA. Hum Mol Genet 7(11):1787–1794 (pii: ddb219)
Jackman HL, Tan FL, Tamei H, Beurling-Harbury C, Li XY, Skidgel RA, Erdos EG (1990) A peptidase in human platelets that deamidates tachykinins. Probable identity with the lysosomal “protective protein”. J Biol Chem 265(19):11265–11272
Seyrantepe V, Hinek A, Peng J, Fedjaev M, Ernest S, Kadota Y, Canuel M, Itoh K, Morales CR, Lavoie J, Tremblay J, Pshezhetsky AV (2008) Enzymatic activity of lysosomal carboxypeptidase (cathepsin) A is required for proper elastic fiber formation and inactivation of endothelin-1. Circulation 117(15):1973–1981
Monti E, Bassi MT, Bresciani R, Civini S, Croci GL, Papini N, Riboni M, Zanchetti G, Ballabio A, Preti A, Tettamanti G, Venerando B, Borsani G (2004) Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics 83(3):445–453
Monti E, Bassi MT, Papini N, Riboni M, Manzoni M, Venerando B, Croci G, Preti A, Ballabio A, Tettamanti G, Borsani G (2000) Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem J 349(Pt 1):343–351
Monti E, Bonten E, D’Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, Tettamanti G (2010) Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem 64:403–479
Monti E, Preti A, Rossi E, Ballabio A, Borsani G (1999) Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases. Genomics 57(1):137–143
Hahn CN, del Pilar Martin M, Schroder M, Vanier MT, Hara Y, Suzuki K, d’Azzo A (1997) Generalized CNS disease and massive GM1-ganglioside accumulation in mice defective in lysosomal acid beta-galactosidase. Hum Mol Genet 6(2):205–211
Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d’Azzo A, Perry VH, Butters TD, Dwek RA, Platt FM (2003) Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain 126(Pt 4):974–987
Wang D, Bonten EJ, Yogalingam G, Mann L, d’Azzo A (2005) Short-term, high dose enzyme replacement therapy in sialidosis mice. Mol Genet Metab 85(3):181–189
Baek RC, Broekman ML, Leroy SG, Tierney LA, Sandberg MA, d’Azzo A, Seyfried TN, Sena-Esteves M (2010) AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS ONE 5(10):e13468
Bonten EJ, Wang D, Toy JN, Mann L, Mignardot A, Yogalingam G, d’Azzo A (2004) Targeting macrophages with baculovirus-produced lysosomal enzymes: implications for enzyme replacement therapy of the glycoprotein storage disorder galactosialidosis. FASEB J 18(9):971–973
Bonten EJ, Yogalingam G, Hu H, Gomero E, van de Vlekkert D, d’Azzo A (2013) Chaperone-mediated gene therapy with recombinant AAV-PPCA in a new mouse model of type I sialidosis. Biochim Biophys Acta 1832(10):1784–1792
Elliot-Smith E, Speak AO, Lloyd-Evans E, Smith DA, van der Spoel AC, Jeyakumar M, Butters TD, Dwek RA, d’Azzo A, Platt FM (2008) Beneficial effects of substrate reduction therapy in a mouse model of GM1 gangliosidosis. Mol Genet Metab 94(2):204–211
Hu H, Gomero E, Bonten E, Gray JT, Allay J, Wu Y, Wu J, Calabrese C, Nienhuis A, d’Azzo A (2012) Preclinical dose-finding study with a liver-tropic, recombinant AAV-2/8 vector in the mouse model of galactosialidosis. Mol Ther 20(2):267–274
Kasperzyk JL, d’Azzo A, Platt FM, Alroy J, Seyfried TN (2005) Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice. J Lipid Res 46(4):744–751
Kasperzyk JL, El-Abbadi MM, Hauser EC, d’Azzo A, Platt FM, Seyfried TN (2004) N-butyldeoxygalactonojirimycin reduces neonatal brain ganglioside content in a mouse model of GM1 gangliosidosis. J Neurochem 89(3):645–653
Leimig T, Mann L, Martin Mdel P, Bonten E, Persons D, Knowles J, Allay JA, Cunningham J, Nienhuis AW, Smeyne R, d’Azzo A (2002) Functional amelioration of murine galactosialidosis by genetically modified bone marrow hematopoietic progenitor cells. Blood 99(9):3169–3178
Sano R, Tessitore A, Ingrassia A, d’Azzo A (2005) Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology. Blood 106(7):2259–2268
Cuervo AM, Mann L, Bonten EJ, d’Azzo A, Dice JF (2003) Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J 22(1):47–59
Lichter-Konecki U, Moter SE, Krawisz BR, Schlotter M, Hipke C, Konecki DS (1999) Expression patterns of murine lysosome-associated membrane protein 2 (Lamp-2) transcripts during morphogenesis. Differentiation 65(1):43–58
Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406(6798):902–906
Cuervo AM, Dice JF (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273(5274):501–503
Arias E, Cuervo AM (2011) Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 23(2):184–189
Kaushik S, Cuervo AM (2012) Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22(8):407–417
Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1(7):570–583
Bossi G, Griffiths GM (2005) CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol 17(1):87–94
Andrews NW (2000) Regulated secretion of conventional lysosomes. Trends Cell Biol 10(8):316–321
Bossi G, Booth S, Clark R, Davis EG, Liesner R, Richards K, Starcevic M, Stinchcombe J, Trambas C, Dell’Angelica EC, Griffiths GM (2005) Normal lytic granule secretion by cytotoxic T lymphocytes deficient in BLOC-1, -2 and -3 and myosins Va, VIIa and XV. Traffic 6(3):243–251
Yogalingam G, Bonten EJ, van de Vlekkert D, Hu H, Moshiach S, Connell SA, d’Azzo A (2008) Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev Cell 15(1):74–86
Stinchcombe J, Bossi G, Griffiths GM (2004) Linking albinism and immunity: the secrets of secretory lysosomes. Science 305(5680):55–59
Kima PE, Burleigh B, Andrews NW (2000) Surface-targeted lysosomal membrane glycoprotein-1 (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell Microbiol 2(6):477–486
Zanoteli E, van de Vlekkert D, Bonten EJ, Hu H, Mann L, Gomero EM, Harris AJ, Ghersi G, d’Azzo A (2010) Muscle degeneration in neuraminidase 1-deficient mice results from infiltration of the muscle fibers by expanded connective tissue. Biochim Biophys Acta 1802(7–8):659–672
Wu X, Steigelman KA, Bonten E, Hu H, He W, Ren T, Zuo J, d’Azzo A (2010) Vacuolization and alterations of lysosomal membrane proteins in cochlear marginal cells contribute to hearing loss in neuraminidase 1-deficient mice. Biochim Biophys Acta 1802(2):259–268
Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K (2008) Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am J Pathol 173(4):1042–1056
Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J (1993) The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest 91(3):1198–1205
Morreau H, Galjart NJ, Gillemans N, Willemsen R, van der Horst GT, d’Azzo A (1989) Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem 264(34):20655–20663
Blanchevoye C, Floquet N, Scandolera A, Baud S, Maurice P, Bocquet O, Blaise S, Ghoneim C, Cantarelli B, Delacoux F, Dauchez M, Efremov RG, Martiny L, Duca L, Debelle L (2013) Interaction between the elastin peptide VGVAPG and human elastin binding protein. J Biol Chem 288(2):1317–1328
Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B (2006) Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem 281(6):3698–3710
Lehman A, Mattman A, Sin D, Pare P, Zong Z, d’Azzo A, Campos Y, Sirrs S, Hinek A (2012) Emphysema in an adult with galactosialidosis linked to a defect in primary elastic fiber assembly. Mol Genet Metab 106(1):99–103
Starcher B, d’Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A (2008) Neuraminidase-1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol 295(4):L637–L647
Miyagi T, Wada T, Iwamatsu A, Hata K, Yoshikawa Y, Tokuyama S, Sawada M (1999) Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J Biol Chem 274(8):5004–5011
Arabkhari M, Bunda S, Wang Y, Wang A, Pshezhetsky AV, Hinek A (2010) Desialylation of insulin receptors and IGF-1 receptors by neuraminidase-1 controls the net proliferative response of L6 myoblasts to insulin. Glycobiology 20(5):603–616
Dridi L, Seyrantepe V, Fougerat A, Pan X, Bonneil E, Thibault P, Moreau A, Mitchell GA, Heveker N, Cairo CW, Issad T, Hinek A, Pshezhetsky AV (2013) Positive regulation of insulin signaling by neuraminidase 1. Diabetes 62(7):2338–2346
Shi J, Wang A, Sen S, Wang Y, Kim HJ, Mitts TF, Hinek A (2012) Insulin induces production of new elastin in cultures of human aortic smooth muscle cells. Am J pathol 180(2):715–726
Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, Pshezhetsky AV (2006) Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem 281(37):27526–27538
Stamatos NM, Carubelli I, van de Vlekkert D, Bonten EJ, Papini N, Feng C, Venerando B, d’Azzo A, Cross AS, Wang LX, Gomatos PJ (2010) LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J Leukoc Biol 88(6):1227–1239
Feng C, Stamatos NM, Dragan AI, Medvedev A, Whitford M, Zhang L, Song C, Rallabhandi P, Cole L, Nhu QM, Vogel SN, Geddes CD, Cross AS (2012) Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS ONE 7(4):e32359
Nan X, Carubelli I, Stamatos NM (2007) Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J Leukoc Biol 81(1):284–296
Amith SR, Jayanth P, Franchuk S, Siddiqui S, Seyrantepe V, Gee K, Basta S, Beyaert R, Pshezhetsky AV, Szewczuk MR (2009) Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj J 26(9):1197–1212
Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR (2010) Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal 22(2):314–324
Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, Guzzo C, Gee K, Beyaert R, Szewczuk MR (2011) Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J Biol Chem 286(42):36532–36549
Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, Nguyen C, Luzina IG, Atamas SP, Passaniti A, Twaddell WS, Puche AC, Wang LX, Cross AS, Goldblum SE (2012) NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J Biol Chem 287(11):8214–8231
Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M (2012) Mechanisms of cancer-associated glycosylation changes. Front Biosci (Landmark Ed) 17:670–699
Adamczyk B, Tharmalingam T, Rudd PM (2011) Glycans as cancer biomarkers. Biochim Biophys Acta 1820(9):134
Harduin-Lepers A, Krzewinski-Recchi MA, Colomb F, Foulquier F, Groux-Degroote S, Delannoy P (2012) Sialyltransferases functions in cancers. Front Biosci (Elite Ed) 4:499–515
Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, Yamanami H, Fujiya T (2008) Human sialidase as a cancer marker. Proteomics 8(16):3303–3311
Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, Sakuraba H, Miyagi T (2009) Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 28(9):1218–1229
Wada T, Hata K, Yamaguchi K, Shiozaki K, Koseki K, Moriya S, Miyagi T (2007) A crucial role of plasma membrane-associated sialidase in the survival of human cancer cells. Oncogene 26(17):2483–2490
Tessitore A, del Pilar Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d’Azzo A (2004) GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell 15(5):753–766
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13(10):1211–1233
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101(5):451–454
Ma Y, Hendershot LM (2001) The unfolding tale of the unfolded protein response. Cell 107(7):827–830
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403(6765):98–103
Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265(13):7248–7256
Ardail D, Popa I, Bodennec J, Louisot P, Schmitt D, Portoukalian J (2003) The mitochondria-associated endoplasmic-reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem J 371:1013–1019
Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382:527–533
Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d’Azzo A (2009) GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 36(3):500–511
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175:901–911
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610
Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262:744–747
Ledeen RW, Wu G (2002) Ganglioside function in calcium homeostasis and signaling. Neurochem Res 27(7–8):637–647
Ledeen RW, Wu G (2006) GM1 ganglioside: another nuclear lipid that modulates nuclear calcium. GM1 potentiates the nuclear sodium-calcium exchanger. Can J Physiol Pharmacol 84:393–402
Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E, Nixon R, d’Azzo A (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nature Commun. doi:10.1038/ncomms3734
Acknowledgments
We thank Dr. Angela McArthur for editing this manuscript. A.d’A. holds the Jewelers for Children Endowed Chair in Genetics and Gene Therapy. Work from d’Azzo’s laboratory that is included in this review was supported by the National Institutes of Health (NIH) grants GM60905 and DK52025, the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC) and the National Tay-Sachs & Allied Disease Association (NTSAD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonten, E.J., Annunziata, I. & d’Azzo, A. Lysosomal multienzyme complex: pros and cons of working together. Cell. Mol. Life Sci. 71, 2017–2032 (2014). https://doi.org/10.1007/s00018-013-1538-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1538-3