Abstract
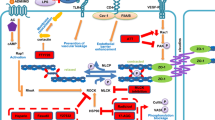
Pseudomonas aeruginosa is a major human opportunistic pathogen and one of the most important causal agents of bacteremia. For non-blood-borne infection, bacterial dissemination requires the crossing of the vascular endothelium, the main barrier between blood and the surrounding tissues. Here, we investigated the effects of P. aeruginosa type 3 secretion effectors, namely ExoS, ExoT, and ExoY, on regulators of actin cytoskeleton dynamics in primary endothelial cells. ExoS and ExoT similarly affected the Lim kinase-cofilin pathway, thereby promoting actin filament severing. Cofilin activation was also observed in a mouse model of P. aeruginosa-induced acute pneumonia. Rho, Rac, and Cdc42 GTPases were sequentially inactivated, leading to inhibition of membrane ruffling, filopodia, and stress fiber collapse, and focal adhesion disruption. At the end of the process, ExoS and ExoT produced a dramatic retraction in all primary endothelial cell types tested and thus a rupture of the endothelial monolayer. ExoY alone had no effect in this context. Cell retraction could be counteracted by overexpression of actin cytoskeleton regulators. In addition, our data suggest that moesin is neither a direct exotoxin target nor an important player in this process. We conclude that any action leading to inhibition of actin filament breakdown will improve the barrier function of the endothelium during P. aeruginosa infection.











Similar content being viewed by others
References
Deng Q, Barbieri JT (2008) Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annu Rev Microbiol 62:271–288
Engel J, Balachandran P (2009) Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol 12(1):61–66
Hauser AR (2009) The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev 7(9):654–665
Vance RE, Rietsch A, Mekalanos JJ (2005) Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73(3):1706–1713
Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT (1999) The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem 274(51):36369–36372
Pederson KJ, Vallis AJ, Aktories K, Frank DW, Barbieri JT (1999) The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol 32(2):393–401
Kazmierczak BI, Engel JN (2002) Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect Immun 70(4):2198–2205
Krall R, Schmidt G, Aktories K, Barbieri JT (2000) Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect Immun 68(10):6066–6068
Barbieri AM, Sha Q, Bette-Bobillo P, Stahl PD, Vidal M (2001) ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect Immun 69(9):5329–5334
Coburn J, Dillon ST, Iglewski BH, Gill DM (1989) Exoenzyme S of Pseudomonas aeruginosa ADP-ribosylates the intermediate filament protein vimentin. Infect Immun 57(3):996–998
Coburn J, Gill DM (1991) ADP-ribosylation of p21ras and related proteins by Pseudomonas aeruginosa exoenzyme S. Infect Immun 59(11):4259–4262
Fraylick JE, Riese MJ, Vincent TS, Barbieri JT, Olson JC (2002) ADP-ribosylation and functional effects of Pseudomonas exoenzyme S on cellular RalA. Biochemistry 41(30):9680–9687
Ganesan AK, Vincent TS, Olson JC, Barbieri JT (1999) Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J Biol Chem 274(31):21823–21829
Henriksson ML, Sundin C, Jansson AL, Forsberg A, Palmer RH, Hallberg B (2002) Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Biochem J 367(Pt 3):617–628
Maresso AW, Baldwin MR, Barbieri JT (2004) Ezrin/radixin/moesin proteins are high affinity targets for ADP-ribosylation by Pseudomonas aeruginosa ExoS. J Biol Chem 279(37):38402–38408
McGuffie EM, Frank DW, Vincent TS, Olson JC (1998) Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun 66(6):2607–2613
Riese MJ, Wittinghofer A, Barbieri JT (2001) ADP ribosylation of Arg41 of Rap by ExoS inhibits the ability of Rap to interact with its guanine nucleotide exchange factor, C3G. Biochemistry 40(11):3289–3294
Rocha CL, Rucks EA, Vincent DM, Olson JC (2005) Examination of the coordinate effects of Pseudomonas aeruginosa ExoS on Rac1. Infect Immun 73(9):5458–5467
Jansson AL, Yasmin L, Warne P, Downward J, Palmer RH, Hallberg B (2006) Exoenzyme S of Pseudomonas aeruginosa is not able to induce apoptosis when cells express activated proteins, such as Ras or protein kinase B/Akt. Cell Microbiol 8(5):815–822
Maresso AW, Deng Q, Pereckas MS, Wakim BT, Barbieri JT (2007) Pseudomonas aeruginosa ExoS ADP-ribosyltransferase inhibits ERM phosphorylation. Cell Microbiol 9(1):97–105
Sun J, Barbieri JT (2003) Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J Biol Chem 278(35):32794–32800
Birge RB, Kalodimos C, Inagaki F, Tanaka S (2009) Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal 7:13
Deng Q, Sun J, Barbieri JT (2005) Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. J Biol Chem 280(43):35953–35960
Phillips RM, Six DA, Dennis EA, Ghosh P (2003) In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem 278(42):41326–41332
Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T (2003) The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin ExoU. EMBO J 22(12):2959–2969
Hritonenko V, Mun JJ, Tam C, Simon NC, Barbieri JT, Evans DJ, Fleiszig SM (2011) Adenylate cyclase activity of Pseudomonas aeruginosa ExoY can mediate bleb-niche formation in epithelial cells and contributes to virulence. Microb Pathog 51(5):305–312
Lee VT, Smith RS, Tummler B, Lory S (2005) Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun 73(3):1695–1705
Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW (1998) ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95(23):13899–13904
Le Berre R, Faure K, Fauvel H, Viget NB, Ader F, Prangere T, Thomas AM, Leroy X, Pittet JF, Marchetti P, Guery BP (2004) Apoptosis inhibition in P. aeruginosa-induced lung injury influences lung fluid balance. Intensive Care Med 30(6):1204–1211
Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, Traber DL (2008) Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med 36(4):1284–1289
Woods DE, Hwang WS, Shahrabadi MS, Que JU (1988) Alteration of pulmonary structure by Pseudomonas aeruginosa exoenzyme S. J Med Microbiol 26(2):133–141
Ganter MT, Roux J, Su G, Lynch SV, Deutschman CS, Weiss YG, Christiaans SC, Myazawa B, Kipnis E, Wiener-Kronish JP, Howard M, Pittet JF (2009) Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am J Respir Cell Mol Biol 40(1):108–118
Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T (2004) Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95(2):196–203
Baldwin MR, Barbieri JT (2005) The type III cytotoxins of yersinia and Pseudomonas aeruginosa that modulate the actin cytoskeleton. Curr Top Microbiol Immunol 291:147–166
Cisz M, Lee PC, Rietsch A (2008) ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol 190(8):2726–2738
Tomar A, Schlaepfer DD (2009) Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 21(5):676–683
Mitra SK, Schlaepfer DD (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18(5):516–523
Zigmond SH (2004) Beginning and ending an actin filament: control at the barbed end. Curr Top Dev Biol 63:145–188
Prudent R, Vassal-Stermann E, Nguyen C, Pillet C, Martinez A, Prunier C, Barette C, Soleilhac E (2012) Pharmacological inhibition of LIM Kinase stabilizes microtubules and inhibits neoplastic growth. Cancer Res 72:4429–4439
Van Aelst L, D’Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11(18):2295–2322
Fehon RG, McClatchey AI, Bretscher A (2011) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11(4):276–287
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG (1999) Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 147(5):1009–1022
Lammers M, Meyer S, Kuhlmann D, Wittinghofer A (2008) Specificity of interactions between mDia isoforms and Rho proteins. J Biol Chem 283(50):35236–35246
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM (2006) Assembly and mechanosensors function of focal adhesions: experiments and models. Eur J Cell Biol 85(3–4):165–173
Galbraith CG, Yamada KM, Sheetz MP (2002) The relationship between force and focal complex development. J Cell Biol 159(4):695–705
Puklin-Faucher E, Sheetz MP (2009) The mechanical integrin cycle. J Cell Sci 122(Pt 2):179–186
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127(5):1015–1026
Barbieri JT, Sun J (2004) Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152:79–92
Fraylick JE, Rucks EA, Greene DM, Vincent TS, Olson JC (2002) Eukaryotic cell determination of ExoS ADP-ribosyltransferase substrate specificity. Biochem Biophys Res Commun 291(1):91–100
Kazmierczak BI, Mostov K, Engel JN (2004) Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa. Mol Biol Cell 15(2):411–419
Mao Y (2011) FORMIN a link between kinetochores and microtubule ends. Trends Cell Biol 21(11):625–629
Mogilner A, Keren K (2009) The shape of motile cells. Curr Biol 19(17):R762–R771
Boyer L, Doye A, Rolando M, Flatau G, Munro P, Gounon P, Clement R, Pulcini C, Popoff MR, Mettouchi A, Landraud L, Dussurget O, Lemichez E (2006) Induction of transient macro apertures in endothelial cells through RhoA inhibition by Staphylococcus aureus factors. J Cell Biol 173(5):809–819
Higgs HN (2001) Actin nucleation: nucleation-promoting factors are not all equal. Curr Biol 11(24):R1009–R1012
Acknowledgments
This work was supported by grants from the Commissariat à l’Energie Atomique, the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique and Joseph Fourier University. Part of this work was also supported by the Alliance pour les sciences de la VIE et de la SANté program in infectiology. We thank Anne-Sophie Ribba for the moesin antibody, Laurence Lafanechère for Pyr1, Cécile Gauthier-Rouvière, Kenzaku Mizuno, Roland Wedlich-Soldner, Hélène Delanoë-Ayari and Gregg Gunderson for expression plasmids, and Arne Rietsch for Pseudomonas aeruginosa mutant strains.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 8 (MPG 842 kb)
Supplementary material 9 (MPG 354 kb)
Supplementary material 10 (MPG 3118 kb)
Rights and permissions
About this article
Cite this article
Huber, P., Bouillot, S., Elsen, S. et al. Sequential inactivation of Rho GTPases and Lim kinase by Pseudomonas aeruginosa toxins ExoS and ExoT leads to endothelial monolayer breakdown. Cell. Mol. Life Sci. 71, 1927–1941 (2014). https://doi.org/10.1007/s00018-013-1451-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1451-9