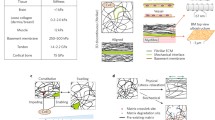
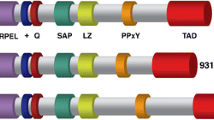
Abstract
Mechanotransduction encompasses the role of mechanical forces in controlling cell behavior by activating signal transduction pathways. Most forces at a cellular level are caused by myosin II, which contracts and cross-links actin. Myosin II-dependent forces are transmitted through the actin cytoskeleton to molecular endpoints that promote specific cellular outcomes, e.g., cell proliferation, adhesion, or migration. For example, most adhesive and migratory phenomena are mechanically linked by a molecular clutch comprised of mechanosensitive scaffolds. Myosin II activation and mechanosensitive molecular mechanisms are finely tuned and spatiotemporally integrated to coordinate morphogenetic events during development. Mechanical events dependent on myosin II also participate in tumor cell proliferation, invasion, and metastatic dissemination. Specifically, tumor cells alter the mechanical properties of the microenvironment to create favorable conditions for proliferation and/or dissemination. These observations position myosin II-dependent force generation and mechanotransduction at the crossroads between normal development and cancer.




Similar content being viewed by others
References
Scott JD, Pawson T (2009) Cell signaling in space and time: where proteins come together and when they’re apart. Science 326(5957):1220–1224. doi:10.1126/science.1175668326/5957/1220
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689. doi:10.1016/j.cell.2006.06.044
Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK (2009) Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19(18):1511–1518. doi:10.1016/j.cub.2009.07.069
Lo CM, Wang HB, Dembo M, Wang YL (2000) Cell movement is guided by the rigidity of the substrate. Biophys J 79(1):144–152
Raab M, Swift J, PC PD, Shah P, Shin JW, Discher DE (2012) Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. doi:10.1083/jcb.201205056
Mooseker MS, Foth BJ (2007) The structural and functional diversity of the myosin family of actin-based molecular motors. In: Coluccio LM (ed) Myosins: a superfamily of molecular motors. Springer, Watertown, pp 1–34
Spudich JA (2001) The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol 2(5):387–392. doi:10.1038/35073086
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299(5613):1743–1747
Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR (2004) Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279(34):35557–35563
Cremo CR, Hartshorne DJ (2007) Smooth-muscle myosin II. In: Coluccio LM (ed) Myosins: a superfamily of molecular motors. Springer, Watertown, pp 171–222
El-Mezgueldi M, Bagshaw CR (2007) The myosin family: biochemical and kinetic properties. In: Coluccio LM (ed) Myosins: a superfamily of molecular motors. Springer, Watertown, pp 55–93
Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR (2006) Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem 281(47):35873–35883
Even-Faitelson L, Ravid S (2006) PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell 17(7):2869–2881
Clark K, Middelbeek J, Dorovkov MV, Figdor CG, Ryazanov AG, Lasonder E, van Leeuwen FN (2008) The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS Lett 582(20):2993–2997
Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN (2008) TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol 378(4):790–803
Luo T, Mohan K, Srivastava V, Ren Y, Iglesias PA, Robinson DN (2012) Understanding the cooperative interaction between myosin II and actin cross-linkers mediated by actin filaments during mechanosensation. Biophys J 102(2):238–247. doi:10.1016/j.bpj.2011.12.020
Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN (2009) Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr Biol 19(17):1421–1428. doi:10.1016/j.cub.2009.07.018
Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, Divi S, Iglesias PA, Kuo SC, Robinson DN (2008) Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol 18(7):471–480
Effler JC, Kee YS, Berk JM, Tran MN, Iglesias PA, Robinson DN (2006) Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr Biol 16(19):1962–1967. doi:10.1016/j.cub.2006.08.027
Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR (2008) Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 10(9):1039–1050. doi:10.1038/ncb1763
Xu XS, Lee E, Chen T, Kuczmarski E, Chisholm RL, Knecht DA (2001) During multicellular migration, myosin ii serves a structural role independent of its motor function. Dev Biol 232(1):255–264
Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10(11):778–790
Conti MA, Adelstein RS (2008) Nonmuscle myosin II moves in new directions. J Cell Sci 121(Pt 1):11–18
Ikebe M (2008) Regulation of the function of mammalian myosin and its conformational change. Biochem Biophys Res Commun 369(1):157–164
Adelstein RS, Conti MA (1975) Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature 256(5518):597–598
Ikebe M, Hartshorne DJ, Elzinga M (1986) Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem 261(1):36–39
Komatsu S, Ikebe M (2007) The phosphorylation of myosin II at the Ser1 and Ser2 Is critical for normal platelet-derived growth factor induced reorganization of myosin filaments. Mol Biol Cell 18(12):5081–5090
Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A (2004) Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J Biol Chem 279(15):14551–14560. doi:10.1074/jbc.M311981200
Vicente-Manzanares M, Cabrero JR, Rey M, Perez-Martinez M, Ursa A, Itoh K, Sanchez-Madrid F (2002) A role for the Rho-p160 Rho coiled-coil kinase axis in the chemokine stromal cell-derived factor-1alpha-induced lymphocyte actomyosin and microtubular organization and chemotaxis. J Immunol 168(1):400–410
Kim KY, Kovacs M, Kawamoto S, Sellers JR, Adelstein RS (2005) Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem 280(24):22769–22775. doi:10.1074/jbc.M503488200
Young PE, Richman AM, Ketchum AS, Kiehart DP (1993) Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev 7(1):29–41
Franke JD, Montague RA, Kiehart DP (2005) Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol 15(24):2208–2221. doi:10.1016/j.cub.2005.11.064
Pouille PA, Ahmadi P, Brunet AC, Farge E (2009) Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal 2 (66):ra16. doi:10.1126/scisignal.2000098
Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153(6):1175–1186
Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO (1986) Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 46(2):271–282. doi:0092-8674(86)90744-0
Horwitz AR (2012) The origins of the molecular era of adhesion research. Nat Rev Mol Cell Biol 13(12):805–811. doi:10.1038/nrm3473
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687. doi:S0092867402009716
Dufour S, Duband JL, Humphries MJ, Obara M, Yamada KM, Thiery JP (1988) Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J 7(9):2661–2671
Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K (1998) Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141(2):539–551
Chrzanowska-Wodnicka M, Burridge K (1996) Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133(6):1403–1415
Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153(4):881–888
Friedland JC, Lee MH, Boettiger D (2009) Mechanically activated integrin switch controls alpha5beta1 function. Science 323(5914):642–644. doi:10.1126/science.1168441
Schurpf T, Springer TA (2011) Regulation of integrin affinity on cell surfaces. EMBO J 30(23):4712–4727. doi:10.1038/emboj.2011.333
Carman CV, Springer TA (2003) Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol 15(5):547–556. doi:S0955067403001121
Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9(8):858–867. doi:10.1038/ncb0807-858
Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K (1986) Interaction of plasma membrane fibronectin receptor with talin–a transmembrane linkage. Nature 320(6062):531–533. doi:10.1038/320531a0
Critchley DR (2009) Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38:235–254. doi:10.1146/annurev.biophys.050708.133744
Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP (2003) Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424(6946):334–337
del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323(5914):638–641. doi:10.1126/science.1162912
Calderwood DA, Yan B, de Pereda JM, Alvarez BG, Fujioka Y, Liddington RC, Ginsberg MH (2002) The phosphotyrosine binding-like domain of talin activates integrins. J Biol Chem 277(24):21749–21758. doi:10.1074/jbc.M111996200
Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA (2010) Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466(7303):263–266. doi:10.1038/nature09198
Sawada Y, Sheetz MP (2002) Force transduction by Triton cytoskeletons. J Cell Biol 156(4):609–615. doi:10.1083/jcb.200110068
Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127(5):1015–1026. doi:10.1016/j.cell.2006.09.044
Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC (2005) Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol 171(2):209–215
Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S (2005) Visualizing the mechanical activation of Src. Nature 434(7036):1040–1045. doi:10.1038/nature03469
Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF (1997) Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385(6616):537–540. doi:10.1038/385537a0
Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1(9):761–772
Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW (2006) Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci 119(Pt 24):5204–5214. doi:10.1242/jcs.03321
Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM (2007) Differential transmission of actin motion within focal adhesions. Science 315(5808):111–115
Jurado C, Haserick JR, Lee J (2005) Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell 16(2):507–518
Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM (2008) Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol 183(6):999–1005
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6(2):154–161
Yamada S, Nelson WJ (2007) Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol 178(3):517–527
Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS (2005) Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell–cell contacts. Mol Biol Cell 16(10):4531–4542
Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS (2004) Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279(40):41263–41266
Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA (2009) Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 17(5):736–743. doi:10.1016/j.devcel.2009.09.003
Keller R, Davidson LA, Shook DR (2003) How we are shaped: the biomechanics of gastrulation. Differentiation 71(3):171–205. doi:10.1046/j.1432-0436.2003.710301.x
Heisenberg CP, Tada M (2002) Zebrafish gastrulation movements: bridging cell and developmental biology. Semin Cell Dev Biol 13(6):471–479. doi:S1084952102001003
Lecuit T, Lenne PF, Munro E (2011) Force generation, transmission, and integration during cell and tissue morphogenesis. Annu Rev Cell Dev Biol 27:157–184. doi:10.1146/annurev-cellbio-100109-104027
Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF (2005) Folded gastrulation, cell shape change and the control of myosin localization. Development 132(18):4165–4178. doi:10.1242/dev.01938
He L, Wang X, Tang HL, Montell DJ (2010) Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol 12(12):1133–1142. doi:10.1038/ncb2124
Simoes S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, Hombria JC, Jacinto A (2006) Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 133(21):4257–4267. doi:10.1242/dev.02588
Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L (2001) Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105(1):81–91
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG (1999) Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 147(5):1009–1022
Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G (2009) Coordination of Rho GTPase activities during cell protrusion. Nature 461(7260):99–103. doi:10.1038/nature08242
Nimnual AS, Taylor LJ, Bar-Sagi D (2003) Redox-dependent downregulation of Rho by Rac. Nat Cell Biol 5(3):236–241. doi:10.1038/ncb938
Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135(3):510–523. doi:10.1016/j.cell.2008.09.043
Bertet C, Sulak L, Lecuit T (2004) Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429(6992):667–671
Zallen JA, Wieschaus E (2004) Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell 6(3):343–355. doi:S1534580704000607
Rauzi M, Verant P, Lecuit T, Lenne PF (2008) Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol 10(12):1401–1410
Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M (2011) A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471(7336):99–103. doi:10.1038/nature09765
Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR (2006) Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 173(4):587–589
Zimmerman SG, Thorpe LM, Medrano VR, Mallozzi CA, McCartney BM (2010) Apical constriction and invagination downstream of the canonical Wnt signaling pathway require Rho1 and myosin II. Dev Biol 340(1):54–66. doi:10.1016/j.ydbio.2010.01.021
Fiehler RW, Wolff T (2007) Drosophila Myosin II, Zipper, is essential for ommatidial rotation. Dev Biol 310(2):348–362. doi:10.1016/j.ydbio.2007.08.001
Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR (2007) Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci USA 104(22):9296–9300. doi:10.1073/pnas.0703153104
Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF (2007) Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 176(5):573–580
Rauzi M, Lenne PF (2011) Cortical forces in cell shape changes and tissue morphogenesis. Curr Top Dev Biol 95:93–144. doi:10.1016/B978-0-12-385065-2.00004-9
Munro E, Nance J, Priess JR (2004) Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell 7(3):413–424. doi:10.1016/j.devcel.2004.08.001
Mayer M, Depken M, Bois JS, Julicher F, Grill SW (2010) Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467(7315):617–621. doi:10.1038/nature09376
Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, Grill SW, Heisenberg CP (2012) Forces driving epithelial spreading in zebrafish gastrulation. Science 338(6104):257–260. doi:10.1126/science.1224143
Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM (2010) Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 26:315–333. doi:10.1146/annurev.cellbio.011209.122036
Butcher DT, Alliston T, Weaver VM (2009) A tense situation: forcing tumour progression. Nat Rev Cancer 9(2):108–122. doi:10.1038/nrc2544
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374
Weinberg R (2006) The biology of cancer. Garland, New York
Schlaepfer DD, Hanks SK, Hunter T, van der Geer P (1994) Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372(6508):786–791
Rivera GM, Antoku S, Gelkop S, Shin NY, Hanks SK, Pawson T, Mayer BJ (2006) Requirement of Nck adaptors for actin dynamics and cell migration stimulated by platelet-derived growth factor B. Proc Natl Acad Sci USA 103(25):9536–9541. doi:10.1073/pnas.0603786103
Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99(19):1441–1454. doi:10.1093/jnci/djm135
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34. doi:10.1007/s10555-006-7886-9
Lucero HA, Kagan HM (2006) Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63(19–20):2304–2316. doi:10.1007/s00018-006-6149-9
Meshel AS, Wei Q, Adelstein RS, Sheetz MP (2005) Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol 7(2):157–164
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254. doi:10.1016/j.ccr.2005.08.010
Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM, Olson MF (2011) Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19(6):776–791. doi:10.1016/j.ccr.2011.05.008
Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, Lim RY, Schoenenberger CA (2012) The nanomechanical signature of breast cancer. Nat Nanotechnol 7(11):757–765. doi:10.1038/nnano.2012.167
de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM (2005) Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171(1):153–164. doi:10.1083/jcb.200506152
Quintin S, Gally C, Labouesse M (2008) Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet 24(5):221–230. doi:10.1016/j.tig.2008.02.005
Friedl P, Wolf K (2010) Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188(1):11–19. doi:10.1083/jcb.200909003
Egeblad M, Rasch MG, Weaver VM (2010) Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 22(5):697–706. doi:10.1016/j.ceb.2010.08.015
Nieto MA, Cano A (2012) The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol 22(5–6):361–368. doi:10.1016/j.semcancer.2012.05.003
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440(7088):1222–1226. doi:10.1038/nature04695
Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, Portillo F, Peinado H, Palacios J, Cano A (2011) Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med 3(9):528–544. doi:10.1002/emmm.201100156
Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J (2005) Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol 5:14. doi:10.1186/1472-6750-5-14
Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ (2005) Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435(7040):365–369. doi:10.1038/nature03550
Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453(7191):51–55. doi:10.1038/nature06887
Bergert M, Chandradoss SD, Desai RA, Paluch E (2012) Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc Natl Acad Sci USA 109(36):14434–14439. doi:10.1073/pnas.12079681091207968109
Maiden SL, Hardin J (2011) The secret life of alpha-catenin: moonlighting in morphogenesis. J Cell Biol 195(4):543–552. doi:10.1083/jcb.201103106
Nikolaidou KK, Barrett K (2004) A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol 14(20):1822–1826. doi:10.1016/j.cub.2004.09.080
Acknowledgments
We apologize for those studies not cited due to space constraints. Also, we are grateful to Professors Michel Labouesse and Francisco Sánchez-Madrid for critical reading of the manuscript. M.V.-M. acknowledges funding from the Ramón y Cajal program (RyC-2010-06094, MINECO, Spain), Grant SAF2011-24953 from the MINECO, Spain, a Marie Curie Career Integration Grant (CIG-293719) and a grant from the Fundación Ramón Areces (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguilar-Cuenca, R., Juanes-García, A. & Vicente-Manzanares, M. Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer. Cell. Mol. Life Sci. 71, 479–492 (2014). https://doi.org/10.1007/s00018-013-1439-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1439-5