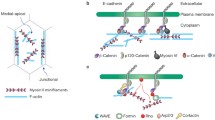
Abstract
Mechanical forces are increasingly recognized as central factors in the regulation of tissue morphogenesis and homeostasis. Central to the transduction of mechanical information into biochemical signaling is the contractile actomyosin cytoskeleton. Fluctuations in actomyosin contraction are sensed by tension sensitive systems at the interface between actomyosin and cell adhesion complexes. We review the current knowledge about the mechanical coupling of cell–cell junctions to the cytoskeleton and highlight the central role of α-catenin in this linkage. We assemble current knowledge about α-catenin’s regulation by tension and about its interactions with a diversity of proteins. We present a model in which α-catenin is a force-regulated platform for a machinery of proteins that orchestrates local cortical remodeling in response to force. Finally, we highlight recently described fundamental processes in tissue morphogenesis and argue where and how this α-catenin-dependent cadherin mechanotransduction may be involved.







Similar content being viewed by others
Abbreviations
- ΔVBS:
-
Delta vinculin binding site
- AJ:
-
Adherens junction
- ARP2/3:
-
Actin-regulated protein
- CAM:
-
Cell adhesion molecule
- Dsc:
-
Desmocollin
- Dsg:
-
Desmoglein
- EC:
-
Extracellular cadherin
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial to mesenchymal transition
- EPLIN:
-
Epithelial protein lost in neoplasia
- F-actin:
-
Filamentous actin
- FAJ:
-
Focal adherens junction
- FAK:
-
Focal adhesion kinase
- FH:
-
Formin homology
- HGF:
-
Hepatocyte growth factor
- JAM:
-
Junctional adhesion molecule
- LAJ:
-
Linear adherens junction
- MTC:
-
Magnetic twisting cytometry
- N-WASP:
-
Wiscott–Aldrich syndrome protein
- TJ:
-
Tight junction
- VASP:
-
Vasodilator-stimulated protein
- YAP:
-
Yes-associated protein
- ZA:
-
Zonula adherens
- ZAJ:
-
Zonula adherens junction
- ZO:
-
Zonula occludens
References
Farquhar MG, Palade GE (1963) Junctional complexes in various epithelia. J Cell Biol 17:375–412
Volk T, Geiger B (1984) A 135-kd membrane protein of intercellular adherens junctions. EMBO J 3(10):2249–2260
Takeichi M (1977) Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol 75(2 Pt 1):464–474
Yoshida-Noro C, Suzuki N, Takeichi M (1984) Molecular nature of the calcium-dependent cell–cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol 101(1):19–27
Peyrieras N et al (1983) Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci USA 80(20):6274–6277
Mege RM et al (1988) Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci USA 85(19):7274–7278
Hulpiau P, van Roy F (2009) Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol 41(2):349–369
Ozawa M, Baribault H, Kemler R (1989) The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 8(6):1711–1717
Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163(3):525–534
Hinck L et al (1994) Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol 125(6):1327–1340
Takahashi K et al (1999) Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol 145(3):539–549
Stevenson BR et al (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103(3):755–766
Gumbiner B, Lowenkopf T, Apatira D (1991) Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA 88(8):3460–3464
Haskins J et al (1998) ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 141(1):199–208
Furuse M et al (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123(6 Pt 2):1777–1788
Saitou M et al (1998) Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141(2):397–408
Furuse M et al (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141(7):1539–1550
Franke WW et al (1981) Antibodies to high molecular weight polypeptides of desmosomes: specific localization of a class of junctional proteins in cells and tissue. Differentiation 20(3):217–241
Franke WW et al (1983) Significance of two desmosome plaque-associated polypeptides of molecular weights 75 000 and 83 000. EMBO J 2(12):2211–2215
Koch PJ et al (1990) Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol 53(1):1–12
Koch PJ et al (1991) Amino acid sequence of bovine muzzle epithelial desmocollin derived from cloned cDNA: a novel subtype of desmosomal cadherins. Differentiation 47(1):29–36
Niessen CM (2007) Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 127(11):2525–2532
Watabe-Uchida M et al (1998) alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol 142(3):847–857
Kametani Y, Takeichi M (2007) Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol 9(1):92–98
Yonemura S et al (1995) Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108(Pt 1):127–142
Sakisaka T et al (2007) The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol 19(5):593–602
Adams CL, Nelson WJ (1998) Cytomechanics of cadherin-mediated cell–cell adhesion. Curr Opin Cell Biol 10(5):572–577
Vasioukhin V et al (2000) Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100(2):209–219
Ayollo DV et al (2009) Rearrangements of the actin cytoskeleton and E-cadherin-based adherens junctions caused by neoplasic transformation change cell–cell interactions. PLoS One 4(11):e8027
de Rooij J et al (2005) Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171(1):153–164
Loerke D et al (2012) Quantitative imaging of epithelial cell scattering identifies specific inhibitors of cell motility and cell–cell dissociation. Sci Signal 5(231):rs5
Taguchi K, Ishiuchi T, Takeichi M (2011) Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol 194(4):643–656
Huveneers S et al (2012) Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 196(5):641–652
Millan J et al (2010) Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol 8:11
Shewan AM et al (2005) Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell–cell contacts. Mol Biol Cell 16(10):4531–4542
Miyake Y et al (2006) Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res 312(9):1637–1650
Abe K, Takeichi M (2008) EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci USA 105(1):13–19
Ratheesh A et al (2012) Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 14(8):818–828
Drees F et al (2005) Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123(5):903–915
Yamada S et al (2005) Deconstructing the cadherin-catenin-actin complex. Cell 123(5):889–901
Ogita H et al (2010) Cell adhesion molecules nectins and associating proteins: implications for physiology and pathology. Proc Jpn Acad B 86(6):621–629
Takai Y et al (2008) The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342
Noren NK et al (2001) Cadherin engagement regulates Rho family GTPases. J Biol Chem 276(36):33305–33308
Sakurai A et al (2006) MAGI-1 is required for Rap1 activation upon cell–cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell 17(2):966–976
McLachlan RW et al (2007) E-cadherin adhesion activates c-Src signaling at cell–cell contacts. Mol Biol Cell 18(8):3214–3223
Kovacs EM et al (2002) Cadherin-directed actin assembly: e-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12(5):379–382
Helwani FM et al (2004) Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol 164(6):899–910
Kovacs EM et al (2011) N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol 13(8):934–943
Kobielak A, Pasolli HA, Fuchs E (2004) Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6(1):21–30
Smutny M et al (2010) Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12(7):696–702
Ratheesh A, Yap AS (2012) A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol 13(10):673–679
Brieher WM, Yap AS (2013) Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 25(1):39–46
Georgiou M et al (2008) Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol 18(21):1631–1638
Angres B, Barth A, Nelson WJ (1996) Mechanism for transition from initial to stable cell–cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biol 134(2):549–557
Lambert M et al (2007) Nucleation and growth of cadherin adhesions. Exp Cell Res 313(19):4025–4040
Krendel M et al (1999) Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell–cell contacts in cultured epitheliocytes. Proc Natl Acad Sci USA 96(17):9666–9670
Gloushankova NA et al (1998) Dynamics of contacts between lamellae of fibroblasts: essential role of the actin cytoskeleton. Proc Natl Acad Sci USA 95(8):4362–4367
Twiss F et al (2012) Vinculin-dependent Cadherin mechanosensing regulates efficient epithelial barrier formation. Biol Open 1(11):1128–1140
le Duc Q et al (2010) Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189(7):1107–1115
Pokutta S et al (2002) Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem 277(21):18868–18874
Tachibana K et al (2000) Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 150(5):1161–1176
Yamamoto T et al (1997) The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol 139(3):785–795
Ooshio T et al (2010) Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J Biol Chem 285(7):5003–5012
Imamura Y et al (1999) Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol 144(6):1311–1322
Rajasekaran AK et al (1996) Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol 132(3):451–463
Mandai K et al (1997) Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 139(2):517–528
Gumbiner B, Stevenson B, Grimaldi A (1988) The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107(4):1575–1587
Ikeda W et al (1999) Afadin: a key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J Cell Biol 146(5):1117–1132
Sawyer JK et al (2011) A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Mol Biol Cell 22(14):2491–2508
Sawyer JK et al (2009) The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol 186(1):57–73
Yonemura S et al (2010) alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12(6):533–542
Kwiatkowski AV et al (2010) In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc Natl Acad Sci USA 107(33):14591–14596
Rangarajan ES, Izard T (2012) The cytoskeletal protein alpha-catenin unfurls upon binding to vinculin. J Biol Chem 287(22):18492–18499
Choi HJ et al (2012) alphaE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci USA 109(22):8576–8581
Nieset JE et al (1997) Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci 110(Pt 8):1013–1022
Huttelmaier S et al (1997) Characterization of two F-actin-binding and oligomerization sites in the cell-contact protein vinculin. Eur J Biochem 247(3):1136–1142
Wen KK, Rubenstein PA, DeMali KA (2009) Vinculin nucleates actin polymerization and modifies actin filament structure. J Biol Chem 284(44):30463–30473
Scott JA et al (2006) Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell 17(3):1085–1095
DeMali KA, Burridge K (2003) Coupling membrane protrusion and cell adhesion. J Cell Sci 116(Pt 12):2389–2397
Otey CA, Carpen O (2004) Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskelet 58(2):104–111
Nola S et al (2011) Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol 195(5):855–871
Dobrosotskaya IY, James GL (2000) MAGI-1 interacts with beta-catenin and is associated with cell–cell adhesion structures. Biochem Biophys Res Commun 270(3):903–909
Cavey M et al (2008) A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453(7196):751–756
Farhadifar R et al (2007) The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr Biol 17(24):2095–2104
Sumida GM et al (2011) Myosin II activity dependent and independent vinculin recruitment to the sites of E-cadherin-mediated cell–cell adhesion. BMC Cell Biol 12:48
Grashoff C et al (2010) Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466(7303):263–266
Borghi N et al (2012) E-cadherin is under constitutive actomyosin-generated tension that is increased at cell–cell contacts upon externally applied stretch. Proc Natl Acad Sci USA 109(31):12568–12573
Weber GF, Bjerke MA, DeSimone DW (2011) Integrins and cadherins join forces to form adhesive networks. J Cell Sci 124(Pt 8):1183–1193
Behrndt M et al (2012) Forces driving epithelial spreading in zebrafish gastrulation. Science 338(6104):257–260
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457
Ng MR et al (2012) Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol 199(3):545–563
Desai RA et al (2009) Cell polarity triggered by cell–cell adhesion via E-cadherin. J Cell Sci 122(Pt 7):905–911
Krens SF, Heisenberg CP (2011) Cell sorting in development. Curr Top Dev Biol 95:189–213
Krieg M et al (2008) Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol 10(4):429–436
Maitre JL et al (2012) Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338(6104):253–256
Martin AC, Kaschube M, Wieschaus EF (2009) Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457(7228):495–499
Martin AC et al (2010) Integration of contractile forces during tissue invagination. J Cell Biol 188(5):735–749
Rauzi M, Lenne PF, Lecuit T (2010) Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468(7327):1110–1114
Solon J et al (2009) Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137(7):1331–1342
He L et al (2010) Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol 12(12):1133–1142
Moore KA et al (2005) Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn 232(2):268–281
Paszek MJ et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Alcaraz J et al (2008) Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J 27(21):2829–2838
Santos OF, Nigam SK (1993) HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol 160(2):293–302
Pozzi A, Zent R (2011) Extracellular matrix receptors in branched organs. Curr Opin Cell Biol 23(5):547–553
Zebda N, Dubrovskyi O, Birukov KG (2012) Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 83(1):71–81
Daley WP, Kohn JM, Larsen M (2011) A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev Dyn 240(9):2069–2083
van Miltenburg MH et al (2009) Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. FASEB J 23(10):3482–3493
Zhang YW, Vande Woude GF (2003) HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem 88(2):408–417
Provenzano PP, Keely PJ (2011) Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci 124(Pt 8):1195–1205
Engler AJ et al (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Dupont S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474(7350):179–183
Cordenonsi M et al (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147(4):759–772
Kim NG et al (2011) E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 108(29):11930–11935
Silvis MR et al (2011) alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4(174):ra33
Schlegelmilch K et al (2011) Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144(5):782–795
Acknowledgments
Johan de Rooij and Floor Twiss were supported by a grant from the Netherlands Scientific Organization (NWO-VIDI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Twiss, F., de Rooij, J. Cadherin mechanotransduction in tissue remodeling. Cell. Mol. Life Sci. 70, 4101–4116 (2013). https://doi.org/10.1007/s00018-013-1329-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1329-x