Abstract
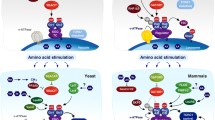
The target of rapamycin (TOR) is a central regulator controlling cell growth. TOR is highly conserved from yeast to mammals, and is deregulated in human cancers and diabetes. TOR complex 1 (TORC1) integrates signals from growth factors, cellular energy status, stress, and amino acids to control cell growth, mitochondrial metabolism, and lipid biosynthesis. The mechanisms of growth factors and cellular energy status in regulating TORC1 have been well established, whereas the mechanism by which amino acid induces TORC1 remains largely unknown. Recent studies revealed that Rag GTPases play a central role in the regulation of TORC1 activation in response to amino acids. In this review, we will discuss the recent progress in our understanding of Rag GTPase-regulated TORC1 activation in response to amino acids. Particular focus will be given to the function of Rag GTPases in TORC1 activation and how Rag GTPases are regulated by amino acids.


Similar content being viewed by others
References
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Yang Q, Guan KL (2007) Expanding mTOR signaling. Cell Res 17(8):666–681
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Kim J, Guan KL (2011) Amino acid signaling in TOR activation. Annu Rev Biochem 80:1001–1032
Russell RC, Fang C, Guan KL (2011) An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development 138(16):3343–3356
Tormos KV, Anso E, Hamanaka RB et al (2011) Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14(4):537–544
Laplante M, Sabatini DM (2009) An emerging role of mTOR in lipid biosynthesis. Curr Biol 19(22):R1046–R1052
Duran RV, Hall MN (2012) Regulation of TOR by small GTPases. EMBO Rep 13(2):121–128
Tee AR, Blenis J (2005) mTOR, translational control and human disease. Semin Cell Dev Biol 16(1):29–37
Inoki K, Corradetti MN, Guan KL (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37(1):19–24
Harrington LS, Findlay GM, Lamb RF (2005) Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30(1):35–42
Polak P, Hall MN (2009) mTOR and the control of whole body metabolism. Curr Opin Cell Biol 21(2):209–218
Dazert E, Hall MN (2011) mTOR signaling in disease. Curr Opin Cell Biol 23(6):744–755
Benjamin D, Colombi M, Moroni C et al (2011) Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 10(11):868–880
Hara K, Yonezawa K, Weng QP et al (1998) Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273(23):14484–14494
Kim E, Goraksha-Hicks P, Li L et al (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10(8):935–945
Sancak Y, Peterson TR, Shaul YD et al (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320(5882):1496–1501
Zoncu R, Bar-Peled L, Efeyan A et al (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334(6056):678–683
Sancak Y, Bar-Peled L, Zoncu R et al (2010) Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141(2):290–303
Gong R, Li L, Liu Y et al (2011) Crystal structure of the Gtr1p–Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev 25(16):1668–1673
Kogan K, Spear ED, Kaiser CA et al (2010) Structural conservation of components in the amino acid sensing branch of the TOR pathway in yeast and mammals. J Mol Biol 402(2):388–398
Binda M, Peli-Gulli MP, Bonfils G et al (2009) The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell 35(5):563–573
Han JM, Jeong SJ, Park MC et al (2012) Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149(2):410–424
Bonfils G, Jaquenoud M, Bontron S et al (2012) Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 46(1):105–110
Kim YM, Stone M, Hwang TH et al (2012) SH3BP4 is a negative regulator of amino acid-rag GTPase-mTORC1 signaling. Mol Cell 46(6):833–846
Heitman J, Movva NR, Hall MN (1991) Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253(5022):905–909
Siekierka JJ, Wiederrecht G, Greulich H et al (1990) The cytosolic-binding protein for the immunosuppressant FK-506 is both a ubiquitous and highly conserved peptidyl-prolyl cis-trans isomerase. J Biol Chem 265(34):21011–21015
Sabatini DM, Erdjument-Bromage H, Lui M et al (1994) RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78(1):35–43
Peng T, Golub TR, Sabatini DM (2002) The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 22(15):5575–5584
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322
Holz MK, Ballif BA, Gygi SP et al (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123(4):569–580
Holz MK, Blenis J (2005) Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280(28):26089–26093
Gingras AC, Gygi SP, Raught B et al (1999) Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13(11):1422–1437
Pause A, Belsham GJ, Gingras AC et al (1994) Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371(6500):762–767
Hara K, Yonezawa K, Kozlowski MT et al (1997) Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem 272(42):26457–26463
Beamish H, Williams R, Chen P et al (1996) Rapamycin resistance in ataxia-telangiectasia. Oncogene 13(5):963–970
Pullen N, Thomas G (1997) The modular phosphorylation and activation of p70s6k. FEBS Lett 410(1):78–82
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318
Harrington LS, Findlay GM, Gray A et al (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166(2):213–223
Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12(Suppl 2):1509–1518
Kamada Y, Funakoshi T, Shintani T et al (2000) Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol 150(6):1507–1513
Hosokawa N, Hara T, Kaizuka T et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20(7):1981–1991
Jung CH, Jun CB, Ro SH et al (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20(7):1992–2003
Ganley IG, du Lam H, Wang J et al (2009) ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284(18):12297–12305
Schieke SM, Finkel T (2006) Mitochondrial signaling, TOR, and life span. Biol Chem 387(10–11):1357–1361
Schieke SM, Phillips D, McCoy JP Jr et al (2006) The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem 281(37):27643–27652
Inoki K, Li Y, Xu T et al (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17(15):1829–1834
Garami A, Zwartkruis FJ, Nobukuni T et al (2003) Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11(6):1457–1466
Tee AR, Manning BD, Roux PP et al (2003) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13(15):1259–1268
Manning BD, Tee AR, Logsdon MN et al (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10(1):151–162
Potter CJ, Pedraza LG, Xu T (2002) Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4(9):658–665
Stocker H, Radimerski T, Schindelholz B et al (2003) Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol 5(6):559–565
Saucedo LJ, Gao X, Chiarelli DA et al (2003) Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol 5(6):566–571
Sancak Y, Thoreen CC, Peterson TR et al (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25(6):903–915
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8(10):774–785
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Inoki K, Ouyang H, Zhu T et al (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126(5):955–968
Corradetti MN, Inoki K, Bardeesy N et al (2004) Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 18(13):1533–1538
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
Brugarolas J, Lei K, Hurley RL et al (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18(23):2893–2904
Reiling JH, Hafen E (2004) The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev 18(23):2879–2892
Ellisen LW, Ramsayer KD, Johannessen CM et al (2002) REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell 10(5):995–1005
Feng Z, Zhang H, Levine AJ et al (2005) The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA 102(23):8204–8209
Stambolic V, MacPherson D, Sas D et al (2001) Regulation of PTEN transcription by p53. Mol Cell 8(2):317–325
Wang X, Campbell LE, Miller CM et al (1998) Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J 334(Pt 1):261–267
Byfield MP, Murray JT, Backer JM (2005) hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280(38):33076–33082
Gulati P, Gaspers LD, Dann SG et al (2008) Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7(5):456–465
Nobukuni T, Kozma SC, Thomas G (2007) hvps34, an ancient player, enters a growing game: mTOR Complex1/S6K1 signaling. Curr Opin Cell Biol 19(2):135–141
Juhasz G, Hill JH, Yan Y et al (2008) The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181(4):655–666
Findlay GM, Yan L, Procter J et al (2007) A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J 403(1):13–20
Yan L, Mieulet V, Burgess D et al (2010) PP2A T61 epsilon is an inhibitor of MAP4K3 in nutrient signaling to mTOR. Mol Cell 37(5):633–642
Yan Y, Flinn RJ, Wu H et al (2009) hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem J 417(3):747–755
Duran A, Amanchy R, Linares JF et al (2011) p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell 44(1):134–146
Pisarewicz K, Mora D, Pflueger FC et al (2005) Polypeptide chains containing d-gamma-hydroxyvaline. J Am Chem Soc 127(17):6207–6215
McArthur KE, Isenberg JI, Hogan DL et al (1983) Intravenous infusion of l-isomers of phenylalanine and tryptophan stimulate gastric acid secretion at physiologic plasma concentrations in normal subjects and after parietal cell vagotomy. J Clin Invest 71(5):1254–1262
Conigrave AD, Quinn SJ, Brown EM (2000) L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA 97(9):4814–4819
San Gabriel A, Uneyama H (2012) Amino acid sensing in the gastrointestinal tract. Amino Acids
Crespo JL, Powers T, Fowler B et al (2002) The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci USA 99(10):6784–6789
Iiboshi Y, Papst PJ, Kawasome H et al (1999) Amino acid-dependent control of p70(s6k). Involvement of tRNA aminoacylation in the regulation. J Biol Chem 274(2):1092–1099
Bun-Ya M, Harashima S, Oshima Y (1992) Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol Cell Biol 12(7):2958–2966
Nakashima N, Hayashi N, Noguchi E et al (1996) Putative GTPase Gtr1p genetically interacts with the RanGTPase cycle in Saccharomyces cerevisiae. J Cell Sci 109(Pt 9):2311–2318
Nakashima N, Noguchi E, Nishimoto T (1999) Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 152(3):853–867
Schurmann A, Brauers A, Massmann S et al (1995) Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J Biol Chem 270(48):28982–28988
Hirose E, Nakashima N, Sekiguchi T et al (1998) RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci 111(Pt 1):11–21
Sekiguchi T, Hirose E, Nakashima N et al (2001) Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem 276(10):7246–7257
Dubouloz F, Deloche O, Wanke V et al (2005) The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell 19(1):15–26
Gao M, Kaiser CA (2006) A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol 8(7):657–667
Nada S, Hondo A, Kasai A et al (2009) The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J 28(5):477–489
Wunderlich W, Fialka I, Teis D et al (2001) A novel 14-kilodalton protein interacts with the mitogen-activated protein kinase scaffold mp1 on a late endosomal/lysosomal compartment. J Cell Biol 152(4):765–776
Kurzbauer R, Teis D, de Araujo ME et al (2004) Crystal structure of the p14/MP1 scaffolding complex: how a twin couple attaches mitogen-activated protein kinase signaling to late endosomes. Proc Natl Acad Sci USA 101(30):10984–10989
Lunin VV, Munger C, Wagner J et al (2004) The structure of the MAPK scaffold, MP1, bound to its partner, p14. A complex with a critical role in endosomal map kinase signaling. J Biol Chem 279(22):23422–23430
Qi J, Wang Y, Forgac M (2007) The vacuolar (H+)-ATPase: subunit arrangement and in vivo regulation. J Bioenerg Biomembr 39(5–6):423–426
Jefferies KC, Forgac M (2008) Subunit H of the vacuolar (H+) ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with the rotary subunit F. J Biol Chem 283(8):4512–4519
Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC et al (2011) Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30(16):3242–3258
Moscat J, Diaz-Meco MT (2009) p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137(6):1001–1004
Proud CG (2011) A new link in the chain from amino acids to mTORC1 activation. Mol Cell 44(1):7–8
Zlatic SA, Tornieri K, L’Hernault SW et al (2011) Metazoan cell biology of the HOPS tethering complex. Cell Logist 1(3):111–117
Zurita-Martinez SA, Puria R, Pan X et al (2007) Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics 176(4):2139–2150
Valbuena N, Guan KL, Moreno S (2012) The Vam6-Gtr1/Gtr2 pathway activates TORC1 in response to amino acids in fission yeast. J Cell Sci
Flinn RJ, Yan Y, Goswami S et al (2010) The late endosome is essential for mTORC1 signaling. Mol Biol Cell 21(5):833–841
Flinn RJ, Backer JM (2010) mTORC1 signals from late endosomes: taking a TOR of the endocytic system. Cell Cycle 9(10):1869–1870
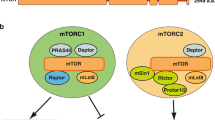
Kim DH, Sarbassov DD, Ali SM et al (2003) GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11(4):895–904
Wullschleger S, Loewith R, Oppliger W et al (2005) Molecular organization of target of rapamycin complex 2. J Biol Chem 280(35):30697–30704
Peterson TR, Laplante M, Thoreen CC et al (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137(5):873–886
Zhao Y, Xiong X, Sun Y (2011) DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell 44(2):304–316
Kim DH, Sarbassov DD, Ali SM et al (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110(2):163–175
Hara K, Maruki Y, Long X et al (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110(2):177–189
Sarbassov DD, Ali SM, Kim DH et al (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14(14):1296–1302
Thedieck K, Polak P, Kim ML et al (2007) PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE 2(11):e1217
Vander Haar E, Lee SI, Bandhakavi S et al (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9(3):316–323
Woo SY, Kim DH, Jun CB et al (2007) PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem 282(35):25604–25612
Pearce LR, Huang X, Boudeau J et al (2007) Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405(3):513–522
Frias MA, Thoreen CC, Jaffe JD et al (2006) mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2 s. Curr Biol 16(18):1865–1870
Yang Q, Inoki K, Ikenoue T et al (2006) Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev 20(20):2820–2832
Acknowledgments
We apologize to those authors whose excellent work we did not reference directly in this review due to the limit of text space. We thank Professor Kun-liang Guan and Dr. Ryan C. Russell at UCSD for critical reading of the manuscript. This work was supported by grants from National Basic Research Program of China (2011CB918600, 2009CB918600) and the National Natural Science Foundation of China (31030019, 11079016, 30870493).
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Yang and R. Gong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, H., Gong, R. & Xu, Y. Control of cell growth: Rag GTPases in activation of TORC1. Cell. Mol. Life Sci. 70, 2873–2885 (2013). https://doi.org/10.1007/s00018-012-1195-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1195-y