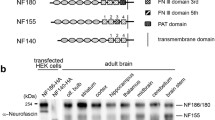
Abstract
Reelin-Disabled-1 (Dab1) signaling has a well-established role in regulating neuronal migration during brain development. Binding of Reelin to its receptors induces Dab1 tyrosine phosphorylation. Tyrosine-phosphorylated Dab1 recruits a wide range of SH2 domain-containing proteins and activates multiple signaling cascades, resulting in cytoskeleton remodeling and precise neuronal positioning. In this review, we summarize recent progress in the Reelin-Dab1 signaling field. We focus on Dab1 alternative splicing as a mechanism for modulating the Reelin signal in developing brain. We suggest that correct positioning of neurons in the developing brain is at least partly controlled by alternatively-spliced Dab1 isoforms that differ in the number and type of tyrosine phosphorylation motifs that they contain. We propose a model whereby different subsets of SH2 domain-containing proteins are activated by different Dab1 isoforms, resulting in coordinated migration of neurons.





Similar content being viewed by others
References
Bellenchi GC et al (2007) N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev 21(18):2347–2357
Chae T et al (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18(1):29–42
Ohshima T et al (1999) Migration defects of cdk5(−/−) neurons in the developing cerebellum is cell autonomous. J Neurosci 19(14):6017–6026
Tsai JW, Bremner KH, Vallee RB (2007) Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci 10(8):970–979
D’Arcangelo G et al (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374(6524):719–723
Howell BW, Herrick TM, Cooper JA (1999) Reelin-induced tryosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev 13(6):643–648
Rice DS, Curran T (2001) Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 24:1005–1039
Pearlman AL et al (1998) New directions for neuronal migration. Curr Opin Neurobiol 8(1):45–54
Gupta A, Tsai LH, Wynshaw-Boris A (2002) Life is a journey: a genetic look at neocortical development. Nat Rev Genet 3(5):342–355
Nadarajah B et al (2003) Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex 13(6):607–611
Nadarajah B, Parnavelas JG (2002) Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci 3(6):423–432
Nadarajah B et al (2001) Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 4(2):143–150
Ayala R, Shu T, Tsai LH (2007) Trekking across the brain: the journey of neuronal migration. Cell 128(1):29–43
Hatanaka Y et al (2004) Distinct migratory behavior of early and late-born neurons derived from the cortical ventricular zone. J Comp Neurol 479(1):1–14
Falconer D (1951) Two mutant mutants, Tremler and Reeler, with neurological actions in the house mouse. J Genet 50:192–201
Caviness VS Jr (1973) Time of neuron origin in the hippocampus and dentate gyrus of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol 151(2):113–120
Caviness VS Jr (1982) Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res 256(3):293–302
Tissir F, Goffinet AM (2003) Reelin and brain development. Nat Rev Neurosci 4(6):496–505
Howell BW et al (1997) Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389(6652):733–737
Sheldon M et al (1997) Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389(6652):730–733
Trommsdorff M et al (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97(6):689–701
D’Arcangelo G et al (1997) Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci 17(1):23–31
D’Arcangelo G et al (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24(2):471–479
Hiesberger T et al (1999) Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24(2):481–489
Herz J, Chen Y (2006) Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7(11):850–859
Chai X et al (2009) Reelin acts as a stop signal for radially migrating neurons by inducing phosphorylation of n-cofilin at the leading edge. Commun Integr Biol 2(4):375–377
Forster E et al (2010) Emerging topics in Reelin function. Eur J Neurosci 31(9):1511–1518
Keshvara L et al (2001) Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J Biol Chem 276(19):16008–16014
Songyang Z et al (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72(5):767–778
Ballif BA et al (2004) Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol 14(7):606–610
Katyal S et al (2007) Hierarchical disabled-1 tyrosine phosphorylation in Src family kinase activation and neurite formation. J Mol Biol 368(2):349–364
Howell BW et al (2000) Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol 10(15):877–885
Feng L et al (2007) Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev 21(21):2717–2730
Feng L, Cooper JA (2009) Dual functions of Dab1 during brain development. Mol Cell Biol 29(2):324–332
Pramatarova A et al (2003) Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol 23(20):7210–7221
Chen K et al (2004) Interaction between Dab1 and CrkII is promoted by Reelin signaling. J Cell Sci 117(Pt 19):4527–4536
Huang Y et al (2004) Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem Biophys Res Commun 318(1):204–212
Assadi AH et al (2003) Interaction of reelin signaling and Lis1 in brain development. Nat Genet 35(3):270–276
Park TJ, Curran T (2008) Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci 28(50):13551–13562
Voss AK et al (2008) C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development 135(12):2139–2149
Jossin Y, Cooper JA (2011) Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 14(6):697–703
Franco SJ et al (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69(3):482–497
Beffert U et al (2002) Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem 277(51):49958–49964
Bock HH et al (2003) Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem 278(40):38772–38779
Ballif BA, Arnaud L, Cooper JA (2003) Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res 117(2):152–159
Jossin Y, Goffinet AM (2007) Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol 27(20):7113–7124
Gonzalez-Billault C et al (2005) A role of MAP1B in Reelin-dependent neuronal migration. Cereb Cortex 15(8):1134–1145
Brich J et al (2003) Genetic modulation of tau phosphorylation in the mouse. J Neurosci 23(1):187–192
Matsuki T et al (2012) Identification of Stk25 as a genetic modifier of Tau phosphorylation in Dab1-mutant mice. PLoS One 7(2):e31152
Matsuki T et al (2010) Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell 143(5):826–836
Chai X et al (2009) Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci 29(1):288–299
Arber S et al (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393(6687):805–809
Yang N et al (1998) Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393(6687):809–812
Leemhuis J et al (2010) Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation. J Neurosci 30(44):14759–14772
Rice DS et al (1998) Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development 125(18):3719–3729
Kuo G et al (2005) Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci 25(37):8578–8586
Arnaud L, Ballif BA, Cooper JA (2003) Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol 23(24):9293–9302
Bock HH et al (2004) Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem 279(32):33471–33479
Simo S, Jossin Y, Cooper JA (2010) Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J Neurosci 30(16):5668–5676
Dulabon L et al (2000) Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27(1):33–44
Sanada K, Gupta A, Tsai LH (2004) Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42(2):197–211
Tabata H, Nakajima K (2002) Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice. J Neurosci Res 69(6):723–730
Magdaleno S, Keshvara L, Curran T (2002) Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron 33(4):573–586
Jossin Y et al (2004) The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci 24(2):514–521
Kubo K et al (2010) Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent “inside-out” alignment in the developing neocortex. J Neurosci 30(33):10953–10966
Olson EC, Kim S, Walsh CA (2006) Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci 26(6):1767–1775
Cooper JA (2008) A mechanism for inside-out lamination in the neocortex. Trends Neurosci 31(3):113–119
Uchida T et al (2009) Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci 29(34):10653–10662
Gao Z et al (2012) Splice-mediated motif switching regulates Disabled-1 phosphorylation and SH2 domain interactions. Mol Cell Biol 32(14):2794–2808
Katyal S, Godbout R (2004) Alternative splicing modulates Disabled-1 (Dab1) function in the developing chick retina. EMBO J 23(8):1878–1888
Gao Z et al (2010) The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina. Mol Cell Biol 30(17):4339–4353
Yano M et al (2010) Nova2 regulates neuronal migration through an RNA switch in disabled-1 signaling. Neuron 66(6):848–858
Bar I et al (2003) The gene encoding disabled-1 (DAB1), the intracellular adaptor of the Reelin pathway, reveals unusual complexity in human and mouse. J Biol Chem 278(8):5802–5812
Costagli A et al (2006) Identification of alternatively spliced dab1 isoforms in zebrafish. Dev Genes Evol 216(6):291–299
Katyal S et al (2011) Disabled-1 alternative splicing in human fetal retina and neural tumors. PLoS One 6(12):e28579
Long H et al (2011) Identification of alternatively spliced Dab1 and Fyn isoforms in pig. BMC Neurosci 12:17
Ule J et al (2006) An RNA map predicting nova-dependent splicing regulation. Nature 444(7119):580–586
Ule J et al (2005) Nova regulates brain-specific splicing to shape the synapse. Nat Genet 37(8):844–852
Hack I et al (2007) Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development 134(21):3883–3891
Ohshima T, Mikoshiba K (2002) Reelin signaling and Cdk5 in the control of neuronal positioning. Mol Neurobiol 26(2–3):153–166
Hashimoto-Torii K et al (2008) Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60(2):273–284
Sibbe M et al (2009) Reelin and Notch1 cooperate in the development of the dentate gyrus. J Neurosci 29(26):8578–8585
Khialeeva E, Lane TF, Carpenter EM (2011) Disruption of reelin signaling alters mammary gland morphogenesis. Development 138(4):767–776
Acknowledgment
This work was supported by the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, Z., Godbout, R. Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage. Cell. Mol. Life Sci. 70, 2319–2329 (2013). https://doi.org/10.1007/s00018-012-1171-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1171-6