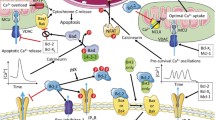
Abstract
Anti-apoptotic Bcl-2-family members not only neutralize pro-apoptotic proteins but also directly regulate intracellular Ca2+ signaling from the endoplasmic reticulum (ER), critically controlling cellular health, survival, and death initiation. Furthermore, distinct Bcl-2-family members may selectively regulate inositol 1,4,5-trisphosphate receptor (IP3R): Bcl-2 likely acts as an endogenous inhibitor of the IP3R, preventing pro-apoptotic Ca2+ transients, while Bcl-XL likely acts as an endogenous IP3R-sensitizing protein promoting pro-survival Ca2+ oscillations. Furthermore, distinct functional domains in Bcl-2 and Bcl-XL may underlie the divergence in IP3R regulation. The Bcl-2 homology (BH) 4 domain, which targets the central modulatory domain of the IP3R, is likely to be Bcl-2’s determining factor. In contrast, the hydrophobic cleft targets the C-terminal Ca2+-channel tail and might be more crucial for Bcl-XL’s function. Furthermore, one amino acid critically different in the sequence of Bcl-2’s and Bcl-XL’s BH4 domains underpins their selective effect on Ca2+ signaling and distinct biological properties of Bcl-2 versus Bcl-XL. This difference is evolutionary conserved across five classes of vertebrates and may represent a fundamental divergence in their biological function. Moreover, these insights open novel avenues to selectively suppress malignant Bcl-2 function in cancer cells by targeting its BH4 domain, while maintaining essential Bcl-XL functions in normal cells. Thus, IP3R-derived molecules that mimic the BH4 domain’s binding site on the IP3R may function synergistically with BH3-mimetic molecules selectivity suppressing Bcl-2’s proto-oncogenic activity. Finally, a more general role for the BH4 domain on IP3Rs, rather than solely anti-apoptotic, may not be excluded as part of a complex network of molecular interactions.



Similar content being viewed by others
References
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18:157–164
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122:437–441
Chipuk JE et al (2010) The BCL-2 family reunion. Mol Cell 37:299–310
Edlich F et al (2011) Bcl-XL retrotranslocates Bax from the mitochondria into the cytosol. Cell 145:104–116
Soriano ME, Scorrano L (2011) Traveling Bax and forth from mitochondria to control apoptosis. Cell 145:15–17
Kim H et al (2009) Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36:487–499
Yao Y, Marassi FM (2009) BAX and BAK caught in the act. Mol Cell 36:353–354
Forte M, Bernardi P (2005) Genetic dissection of the permeability transition pore. J Bioenerg Biomembr 37:121–128
Baumgartner HK et al (2009) Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem 284:20796–20803
Roy SS et al (2009) Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell 33:377–388
Deng J et al (2007) BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12:171–185
Certo M et al (2006) Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9:351–365
Chen L et al (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17:393–403
Kim H et al (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8:1348–1358
Kuwana T et al (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525–535
Opferman JT et al (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671–676
Del Gaizo Moore V et al (2007) Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117:112–121
Zhang L, Ming L, Yu J (2007) BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat 10:207–217
Park CM et al (2008) Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem 51:6902–6915
Tse C et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428
Baffy G et al (1993) Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem 268:6511–6519
Lam M et al (1994) Evidence that Bcl-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Nat Acad Sci USA 91:6569–6573
Thomenius MJ, Distelhorst CW (2003) Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J Cell Sci 116:4493–4499
Rong Y, Distelhorst CW (2008) Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 70:73–91
Pinton P, Rizzuto R (2006) Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ 13:1409–1418
De Smedt H, Verkhratsky A, Muallem S (2011) Ca2+ signaling mechanisms of cell survival and cell death: an introduction. Cell Calcium 50:207–210
Zhivotovsky B, Orrenius S (2011) Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 50:211–221
Romagnoli A et al (2007) Endoplasmic reticulum/mitochondria calcium cross-talk. Novartis Found Symp 287:122–131 (discussion 131–9)
Zecchini E et al (2007) Mitochondrial calcium signalling: message of life and death. Ital J Biochem 56:235–242
Giorgi C et al (2008) Ca2+ signaling, mitochondria and cell death. Curr Mol Med 8:119–130
Pinton P et al (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27:6407–6418
Giorgi C et al (2009) Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol 41:1817–1827
Rizzuto R et al (2009) Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787:1342–1351
Verfaillie T et al (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS based ER stress. Cell Death Differ. doi:10.1038/cdd.2012.74
Pinton P et al (2001) The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J 20:2690–2701
Cardenas C et al (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142:270–283
Higo T et al (2010) Mechanism of ER stress-induced brain damage by IP3 receptor. Neuron 68:865–878
Criollo A et al (2007) Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ 14:1029–1039
Vicencio JM et al (2009) The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ 16:1006–1017
Decuypere JP et al (2011) IP 3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy 7:1472–1489
Decuypere JP, Bultynck G, Parys JB (2011) A dual role for Ca2+ in autophagy regulation. Cell Calcium 50:242–250
De Stefani D et al (2012) VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19:267–273
Baughman JM et al (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345
De Stefani D et al (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340
Jean-Quartier C et al (2012) Studying mitochondrial Ca2+ uptake: a revisit. Mol Cell Endocrinol 353:114–127
Sammels E et al (2010) Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium 47:297–314
Decuypere JP et al (2011) The IP3 receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta 1813:1003–1013
Decuypere JP et al (2011) IP3 receptors, mitochondria, and Ca signaling: implications for aging. J Aging Res 2011:920178
Mekahli D et al (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3:1–30. pii:a004317
Marchi S et al (2012) Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis 3:e304
Szado T et al (2008) Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Nat Acad Sci USA 105:2427–2432
Carnero A (2010) The PKB/AKT pathway in cancer. Curr Pharm Des 16:34–44
Giorgi C et al (2010) PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330:1247–1251
Jones AW, Szabadkai G (2010) Ca2+ transfer from the ER to mitochondria: channeling cell death by a tumor suppressor. Dev Cell 19:789–790
Pinton P, Giorgi C, Pandolfi PP (2011) The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ 18:1450–1456
Rimessi A et al (2009) Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc Nat Acad Sci USA 106:12753–12758
Arbel N, Shoshan-Barmatz V (2010) Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem 285:6053–6062
Palmer AE et al (2004) Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Nat Acad Sci USA 101:17404–17409
Kim HR et al (2008) Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem 283:15946–15955
Xu C et al (2008) BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem 283:11477–11484
Bultynck G et al (2012) The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J Biol Chem 287:2544–2557
Kiviluoto S et al (2012) Bax Inhibitor-1 is a novel IP3 receptor-interacting and-sensitizing protein. Cell Death Dis 3:e367
Sano R et al (2012) Endoplasmic reticulum protein BI-1 regulates Ca2+-mediated bioenergetics to promote autophagy. Genes Dev 26:1041–1054
Pinton P et al (2000) Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol 148:857–862
Scorrano L et al (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Oakes SA et al (2005) Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Nat Acad Sci USA 102:105–110
He H et al (1997) Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol 138:1219–1228
Erin N, Billingsley ML (2004) Domoic acid enhances Bcl-2-calcineurin-inositol-1,4,5-trisphosphate receptor interactions and delayed neuronal death in rat brain slices. Brain Res 1014:45–52
Erin N, Bronson SK, Billingsley ML (2003) Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue. Neuroscience 117:541–555
Xu L et al (2007) Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1. Mol Cell Biochem 295:153–165
Chen R et al (2004) Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 166:193–203
White C et al (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol 7:1021–1028
Eckenrode EF et al (2010) Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem 285:13678–13684
Li C et al (2002) Bcl-XL affects Ca2+ homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc Nat Acad Sci USA 99:9830–9835
Kuo TH et al (1998) Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene 17:1903–1910
Kobrinsky EM, Kirchberger MA (2001) Evidence for a role of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in thapsigargin and Bcl-2 induced changes in Xenopus laevis oocyte maturation. Oncogene 20:933–941
Vento MT et al (2010) Praf2 is a novel Bcl-XL/Bcl-2 interacting protein with the ability to modulate survival of cancer cells. PLoS One 5:e15636
Dremina ES et al (2004) Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). Biochem J 383:361–370
Dremina ES, Sharov VS, Schoneich C (2006) Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry 45:175–184
Dremina ES, Sharov VS, Schoneich C (2012) Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+-mediated apoptosis. Biochem J 444:127–139
Ahmad S et al (2009) Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am J Respir Crit Care Med 179:816–826
Broker LE, Kruyt FA, Giaccone G (2005) Cell death independent of caspases: a review. Clin Cancer Res 11:3155–3162
Galluzzi L et al (2012) Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death. Cell Death Differ 19:107–120
Sperandio S, de Belle I, Bredesen DE (2000) An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA 97:14376–14381
Ladasky JJ et al (2006) Bap31 enhances the endoplasmic reticulum export and quality control of human class I MHC molecules. J Immunol 177:6172–6181
Wang B et al (2008) BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 133:1080–1092
Wang B et al (2003) Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria. J Biol Chem 278:14461–14468
Breckenridge DG et al (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160:1115–1127
Heath-Engel HM, Wang B, Shore GC (2012) Bcl2 at the endoplasmic reticulum protects against a Bax/Bak-independent paraptosis-like cell death pathway initiated via p20Bap31. Biochim Biophys Acta 1823:335–347
Lur G et al (2009) Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP3 receptors. Curr Biol 19:1648–1653
Ferdek PE et al (2012) A novel role for Bcl-2 in regulation of cellular calcium extrusion. Curr Biol 22:1241–1246
Gerasimenko J et al (2010) Inhibitors of Bcl-2 protein family deplete ER Ca2+ stores in pancreatic acinar cells. Pflugers Arch 460:891–900
Zhong F et al (2006) Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J Cell Biol 172:127–137
Hanson CJ et al (2008) Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium 44:324–338
Rong YP et al (2008) Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell 31:255–265
Rong YP et al (2009) The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Nat Acad Sci USA 106:14397–14402
Distelhorst CW, Bootman MD (2011) Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca(2+) signaling and disease. Cell Calcium 50:234–241
Li C et al (2007) Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Nat Acad Sci USA 104:12565–12570
Chan J et al (2010) Structural studies of inositol 1,4,5-trisphosphate receptor: coupling ligand binding to channel gating. J Biol Chem 285:36092–36099
Foskett JK et al (2009) Bcl-xL regulation of InsP3 receptor gating mediated by dual Ca2+ release channel BH3 domains. Biophys J 96:391a
Monaco G et al (2012) Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ 19:295–309
Rong YP et al (2009) Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochim Biophys Acta 1793:971–978
Zhong F et al (2011) Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood 117:2924–2934
Popgeorgiev N et al (2011) The apoptotic regulator Nrz controls cytoskeletal dynamics via the regulation of Ca2+ trafficking in the zebrafish blastula. Dev Cell 20:663–676
Solnica-Krezel L (2006) Gastrulation in zebrafish: all just about adhesion? Curr Opin Genet Dev 16:433–441
Arnaud E et al (2006) The zebrafish bcl-2 homologue Nrz controls development during somitogenesis and gastrulation via apoptosis-dependent and -independent mechanisms. Cell Death Differ 13:1128–1137
Bonneau B et al (2011) Cytoskeleton dynamics in early zebrafish development: a matter of phosphorylation? BioArchitecture 1:1–5
Allen DG et al (2010) Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88:83–91
Basset O et al (2006) Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem J 395:267–276
Velmurugan GV, C White (2011) Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol 302(1):H124–H134
Machado-Vieira R et al (2011) The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biol Psychiatry 69:344–352
Uemura T et al (2011) Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder. Bipolar Disord 13:41–51
Salvadore G et al (2009) Bcl-2 polymorphism influences gray matter volume in the ventral striatum in healthy humans. Biol Psychiatry 66:804–807
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159
Lock R et al (2008) Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer 50:1181–1189
Vogler M et al (2009) Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ 16:360–367
Azmi AS, Mohammad RM (2009) Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J Cell Physiol 218:13–21
High LM et al (2010) The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol 77:483–494
Ackler S et al (2010) The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol 66:869–880
Vogler M et al (2011) BCL2/BCL-XL inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 117:7145–7154
Schoenwaelder SM et al (2011) Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118:1663–1674
Mason KD et al (2007) Programmed anuclear cell death delimits platelet life span. Cell 128:1173–1186
Eno CO et al (2012) Distinct roles of mitochondria- and ER-localized Bcl-xL in apoptosis resistance and Ca2+ homeostasis. Mol Biol Cell 23:2605–2618
Haughn L et al (2003) BCL-2 and BCL-XL restrict lineage choice during hematopoietic differentiation. J Biol Chem 278:25158–25165
Katz C et al (2008) Molecular basis of the interaction between the antiapoptotic Bcl-2 family proteins and the proapoptotic protein ASPP2. Proc Natl Acad Sci USA 105:12277–12282
Shimizu S et al (2000) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci USA 97:3100–3105
Shoshan-Barmatz V et al (2006) The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des 12:2249–2270
Shoshan-Barmatz V et al (2010) VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31:227–285
Zaid H et al (2005) The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ 12:751–760
Abu-Hamad S, Sivan S, Shoshan-Barmatz V (2006) The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci USA 103:5787–5792
Tornero D, Posadas I, Cena V (2011) Bcl-x(L) blocks a mitochondrial inner membrane channel and prevents Ca2+ overload-mediated cell death. PLoS One 6:e20423
Keinan N, Tyomkin D, Shoshan-Barmatz V (2010) Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol 30:5698–5709
Abu-Hamad S et al (2008) Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem 283:13482–13490
Abu-Hamad S et al (2009) The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci 122:1906–1916
Geula S, Ben-Hail D, Shoshan-Barmatz V (2012) Structure-based analysis of VDAC1: n-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem J 444:475–485
Malia TJ, Wagner G (2007) NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry 46:514–525
Xu Q, Reed JC (1998) Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell 1:337–346
Chae HJ et al (2004) BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell 15:355–366
Ahn T et al (2010) Cardiolipin, phosphatidylserine, and BH4 domain of Bcl-2 family regulate Ca2+/H+ antiporter activity of human Bax inhibitor-1. Cell Calcium 47:387–396
Westphalen BC et al (2005) BI-1 protects cells from oxygen glucose deprivation by reducing the calcium content of the endoplasmic reticulum. Cell Death Differ 12:304–306
Henke N et al (2011) The ancient cell death suppressor BAX inhibitor-1. Cell Calcium 50:251–260
de Mattia F et al (2009) Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell 20:3638–3645
Rojas-Rivera D et al (2012) TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell Death Differ 19:1013–1016
Sullivan A, Lu X (2007) ASPP: a new family of oncogenes and tumour suppressor genes. Br J Cancer 96:196–200
Vives V, Slee EA, Lu X (2006) ASPP2: a gene that controls life and death in vivo. Cell Cycle 5:2187–2190
Benyamini H, Friedler A (2011) The ASPP interaction network: electrostatic differentiation between pro- and anti-apoptotic proteins. J Mol Recognit 24:266–274
Kampa KM, Bonin M, Lopez CD (2009) New insights into the expanding complexity of the tumor suppressor ASPP2. Cell Cycle 8:2871–2876
Ahn J et al (2009) Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem 284:13812–13822
Benyamini H et al (2009) A model for the interaction between NF-kappa-B and ASPP2 suggests an I-kappa-B-like binding mechanism. Proteins 77:602–611
Rotem S et al (2008) The structure and interactions of the proline-rich domain of ASPP2. J Biol Chem 283:18990–18999
Rotem S, Katz C, Friedler A (2007) Insights into the structure and protein-protein interactions of the pro-apoptotic protein ASPP2. Biochem Soc Trans 35:966–969
Díez J, Walter D, Muñoz-Pinedo C, Gabaldón T (2010) DeathBase: a database on structure, evolution and function of proteins involved in apoptosis and other forms of cell death. Cell Death Differ 17:735–736
Acknowledgments
Work in the author’s laboratory has been supported by research grants from the Research Council of the KU Leuven (OT/STRT1/10/044), from the Research Foundation—Flanders (FWO) grants G.0788.11 and G.0571.12, and from the Royal Flemish Academy of Belgium for Science and the Arts (Research Award from the Octaaf Dupont Foundation 2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Monaco, T. Vervliet and H. Akl contributed equally to this work.
Rights and permissions
About this article
Cite this article
Monaco, G., Vervliet, T., Akl, H. et al. The selective BH4-domain biology of Bcl-2-family members: IP3Rs and beyond. Cell. Mol. Life Sci. 70, 1171–1183 (2013). https://doi.org/10.1007/s00018-012-1118-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1118-y