Abstract
Insulin/IGF-like signaling regulates the development, growth, fecundity, metabolic homeostasis, stress resistance and lifespan in worms, flies and mammals. Eight insulin-like peptides (DILP1-8) are found in Drosophila. Three of these (DILP2, 3 and 5) are produced by a set of median neurosecretory cells (insulin-producing cells, IPCs) in the brain. Activity in the IPCs of adult flies is regulated by glucose and several neurotransmitters and neuropeptides. One of these, short neuropeptide F (sNPF), regulates food intake, growth and Dilp transcript levels in IPCs via the sNPF receptor (sNPFR1) expressed on IPCs. Here we identify a set of brain neurons that utilizes sNPF to activate the IPCs. These sNPF-expressing neurons (dorsal lateral peptidergic neurons, DLPs) also produce the neuropeptide corazonin (CRZ) and have axon terminations impinging on IPCs. Knockdown of either sNPF or CRZ in DLPs extends survival in flies exposed to starvation and alters carbohydrate and lipid metabolism. Expression of sNPF in DLPs in the sNPF mutant background is sufficient to rescue wild-type metabolism and response to starvation. Since CRZ receptor RNAi in IPCs affects starvation resistance and metabolism, similar to peptide knockdown in DLPs, it is likely that also CRZ targets the IPCs. Knockdown of sNPF, but not CRZ in DLPs decreases transcription of Dilp2 and 5 in the brain, suggesting different mechanisms of action on IPCs of the two co-released peptides. Our findings indicate that sNPF and CRZ co-released from a small set of neurons regulate IPCs, stress resistance and metabolism in adult Drosophila.









Similar content being viewed by others
References
Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R et al (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11:213–221
Antonova Y, Arik AJ, Moore W, Riehle MR, Brown MR (2012) Insulin-like peptides: structure, signaling, and function. In: Gilbert LI (ed) Insect endocrinology. Elsevier/Academic Press, New York, pp 63–92
Giannakou ME, Partridge L (2007) Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci 32:180–188
Géminard G, Arquier N, Layalle S, Bourouis M, Slaidina M et al (2006) Control of metabolism and growth through insulin-like peptides in Drosophila. Diabetes 55:S5–S8
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328:321–326
Baker KD, Thummel CS (2007) Diabetic larvae and obese flies—emerging studies of metabolism in Drosophila. Cell Metab 6:257–266
Broughton S, Alic N, Slack C, Bass T, Ikeya T et al (2008) Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One 3:e3721
Grönke S, Clarke DF, Broughton S, Andrews TD, Partridge L (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6:e1000857
Colombani J, Andersen DS, Leopold P (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336:582–585
Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M (2012) Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336:579–582
Cao C, Brown MR (2001) Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304:317–321
Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296:1118–1120
Cognigni P, Bailey AP, Miguel-Aliaga I (2011) Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13:92–104
Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J et al (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102:3105–3110
Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM et al (2010) DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell 9:336–346
Karpac J, Hull-Thompson J, Falleur M, Jasper H (2009) JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell 8:288–295
Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C et al (2009) Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 1:699–713
Kreneisz O, Chen X, Fridell YW, Mulkey DK (2010) Glucose increases activity and Ca(2+) in insulin-producing cells of adult Drosophila. NeuroReport 21:1116–1120
Ashcroft FM, Gribble FM (1999) ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 42:903–919
Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10:199–207
Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J et al (2003) A nutrient sensor mechanism controls Drosophila growth. Cell 114:739–749
Lee KS, You KH, Choo JK, Han YM, Yu K (2004) Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem 279:50781–50789
Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ et al (2008) Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol 10:468–475
Kaplan DD, Zimmermann G, Suyama K, Meyer T, Scott MP (2008) A nucleostemin family GTPase, NS3, acts in serotonergic neurons to regulate insulin signaling and control body size. Genes Dev 22:1877–1893
Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep: wake behavior. Neuron 65:670–681
Birse RT, Söderberg JA, Luo J, Winther ÅM, Nässel DR (2011) Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol 214:4201–4208
Enell LE, Kapan N, Söderberg JA, Kahsai L, Nässel DR (2010) Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila. PLoS One 5:e15780
Luo J, Becnel J, Nichols CD, Nässel DR (2012) Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT(1A) receptor. Cell Mol Life Sci 69:471–484
Lee KS, Hong SH, Kim AK, Ju SK, Kwon OY et al (2009) Processed short neuropeptide F peptides regulate growth through the ERK-insulin pathway in Drosophila melanogaster. FEBS Lett 583:2573–2577
Feng G, Reale V, Chatwin H, Kennedy K, Venard R et al (2003) Functional characterization of a neuropeptide F-like receptor from Drosophila melanogaster. Eur J Neurosci 18:227–238
Mertens I, Meeusen T, Huybrechts R, De Loof A, Schoofs L (2002) Characterization of the short neuropeptide F receptor from Drosophila melanogaster. Biochem Biophys Res Commun 297:1140–1148
Nässel DR, Enell LE, Santos JG, Wegener C, Johard HA (2008) A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci 9:90
Hewes RS, Taghert PH (2001) Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res 11:1126–1142
Park Y, Kim YJ, Adams ME (2002) Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA 99:11423–11428
Cazzamali G, Saxild N, Grimmelikhuijzen C (2002) Molecular cloning and functional expression of a Drosophila corazonin receptor. Biochem Biophys Res Commun 298:31–36
Veenstra JA (2009) Does corazonin signal nutritional stress in insects? Insect Biochem Mol Biol 39:755–762
Boerjan B, Verleyen P, Huybrechts J, Schoofs L, De Loof A (2010) In search for a common denominator for the diverse functions of arthropod corazonin: a role in the physiology of stress? Gen Comp Endocrinol 166:222–233
Zhao Y, Bretz CA, Hawksworth SA, Hirsh J, Johnson EC (2010) Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS One 5:e9141
Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA et al (2005) A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol 208:1239–1246
Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA et al (2002) The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol 205:3799–3807
Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557
Li XF, Bowe JE, Mitchell JC, Brain SD, Lightman SL et al (2004) Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: a novel neural action for calcitonin gene-related peptide. Endocrinology 145:1556–1563
Li XF, Bowe JE, Lightman SL, O’Byrne KT (2005) Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology 146:318–322
Kahsai L, Carlsson MA, Winther ÅM, Nässel DR (2012) Distribution of metabotropic receptors of serotonin, dopamine, GABA, glutamate, and short neuropeptide F in the central complex of Drosophila. Neuroscience 208:11–26
Choi SH, Lee G, Monahan P, Park JH (2008) Spatial regulation of Corazonin neuropeptide expression requires multiple cis-acting elements in Drosophila melanogaster. J Comp Neurol 507:1184–1195
Lee G, Park JH (2004) Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167:311–323
Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12:1293–1300
Nitabach MN, Blau J, Holmes TC (2002) Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109:485–495
Johard HA, Enell LE, Gustafsson E, Trifilieff P, Veenstra JA et al (2008) Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: relations to extrinsic neurons expressing different neurotransmitters. J Comp Neurol 507:1479–1496
Fernandez-Ayala DJ, Sanz A, Vartiainen S, Kemppainen KK, Babusiak M et al (2009) Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab 9:449–460
Vanden Broeck J (2001) Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides 22:241–254
Nässel DR, Wegener C (2011) A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling? Peptides 32:1335–1355
Choi YJ, Lee G, Hall JC, Park JH (2005) Comparative analysis of Corazonin-encoding genes (Crz’s) in Drosophila species and functional insights into Crz-expressing neurons. J Comp Neurol 482:372–385
Veenstra JA (1994) Isolation and structure of the Drosophila corazonin gene. Biochem Biophys Res Commun 204:292–296
Yew JY, Wang Y, Barteneva N, Dikler S, Kutz-Naber KK et al (2009) Analysis of neuropeptide expression and localization in adult Drosophila melanogaster central nervous system by affinity cell-capture mass spectrometry. J Proteome Res 8:1271–1284
Cantera R, Veenstra JA, Nässel DR (1994) Postembryonic development of corazonin-containing neurons and neurosecretory cells in the blowfly, Phormia terraenovae. J Comp Neurol 350:559–572
Lee G, Kim KM, Kikuno K, Wang Z, Choi YJ et al (2008) Developmental regulation and functions of the expression of the neuropeptide corazonin in Drosophila melanogaster. Cell Tissue Res 331:659–673
Siegmund T, Korge G (2001) Innervation of the ring gland of Drosophila melanogaster. J Comp Neurol 431:481–491
Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S et al (2008) Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA 105:9715–9720
Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39:715–720
Bharucha KN, Tarr P, Zipursky SL (2008) A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J Exp Biol 211:3103–3110
Teleman AA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425:13–26
Kim YJ, Spalovska-Valachova I, Cho KH, Zitnanova I, Park Y et al (2004) Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA 101:6704–6709
Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG et al (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol 447:366–380
Tawfik AI, Tanaka S, De Loof A, Schoofs L, Baggerman G et al (1999) Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc Natl Acad Sci USA 96:7083–7087
Predel R, Neupert S, Russell WK, Scheibner O, Nachman RJ (2007) Corazonin in insects. Peptides 28:3–10
Veenstra JA (1989) Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett 250:231–234
Hillyer JF, Estevez-Lao TY, Funkhouser LJ, Aluoch VA (2012) Anopheles gambiae corazonin: gene structure, expression and effect on mosquito heart physiology. Insect Mol Biol 21:343–355
Bergland AO, Chae HS, Kim YJ, Tatar M (2012) Fine-scale mapping of natural variation in fly fecundity identifies neuronal domain of expression and function of an aquaporin. PLoS Genet 8:e1002631
Melcher C, Pankratz MJ (2005) Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 3:e305
Staubli F, Jorgensen TJ, Cazzamali G, Williamson M, Lenz C et al (2002) Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci USA 99:3446–3451
Veenstra JA, Agricola HJ, Sellami A (2008) Regulatory peptides in fruit fly midgut. Cell Tissue Res 334:499–516
Yoon JG, Stay B (1995) Immunocytochemical localization of Diploptera punctata allatostatin-like peptide in Drosophila melanogaster. J Comp Neurol 363:475–488
Hergarden AC, Tayler TD, Anderson DJ (2012) Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci USA 109:3967–3972
Acknowledgments
We thank S. Broughton, E. Hafen, P. Léopold, J.H. Park, J.A. Veenstra, K. Yu, the Bloomington Drosophila Stock Center, the Harvard Stock Center, and the Vienna Drosophila RNAi Center for flies and reagents. Å.M.E. Winther is gratefully acknowledged for technical advice. This study was supported by the Swedish Research Council (VR) and the Carl Trygger Foundation (both to DRN).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2012_1097_MOESM1_ESM.tif
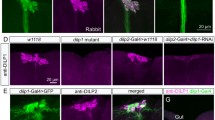
S Fig 1 In male flies there is a small set of Crz-Gal4 expressing neurons in the last abdominal segment that do not produce sNPF. As seen in a - c Crz-Gal4 and sNPF expressing neurons in abdominal ganglia are distinct. Thus the use of the Crz-Gal4 driver to interfere with sNPF expression will only affect the two sets of DLPs in the brain. (TIFF 1,941 kb)
18_2012_1097_MOESM2_ESM.tif
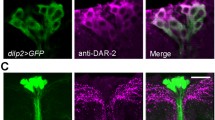
S Fig 2 Morphological details of superposition of DLPs and IPCs in the Drosophila brain. a The DLPs are displayed with Crz-Gal4-driven GFP (green) and IPCs with DILP2 immunolabeling (magenta). The DLPs send axons along the median bundle (MB) and posterior lateral tract (PLT), whereas the axons of the IPCs run in the MB. b Higher magnification of the same neurons showing the tight spatial relations between axon branches of the two neuron types in the median bundle. The region indicated with double arrow (Dendr) is where the IPCs (and DLPs to a lesser extent) have short lateral processes. c1 - c3 Details of the region indicated by double arrow in 1b. Note that both Crz-Gal4 (green) and DILP2 immunolabeled (magenta) axons have short side branches in this region and these may represent functional contacts between the two neuron types. d1 - d2 Another region where DLPs may contact IPCs is in the tritocerebrum (TC) on either side of the esophageal foramen (EF). Clearly the DLPs arborize in additional areas ventral to the TC, including the subesophageal ganglion, suggesting additional outputs and/or inputs. (TIFF 4,089 kb)
18_2012_1097_MOESM3_ESM.tif
S Fig 3 Corazonin/sNPF, DILP2 and sNPFR1-expressing neurons in the third instar larva. a - c Crz-Gal4 (green) and DILP2 immunreactive neurons (aDILP2; magenta) in the larval brain and ring gland (RG). The DLPs and IPCs have processes that superimpose in the corpora cardiaca (CC) of the ring gland and in portions of the brain. For some reason the cell bodies of the mushroom body Kenyon cells (asterisk) display non-specific immunolabeling with anti-DILP2. d Detail of a ring gland where DLP and IPC processes superimpose in the CC, but not corpora allata (CA) of the ring gland (encircled). e - h Using the snpfr-Gal4 driver it appears that the IPCs (labeled with anti-DILP2) do not express the sNPF receptor in the larva. The "white cells" seen in g are above each other in this image stack and do not colocalize the markers. (TIFF 2,398 kb)
18_2012_1097_MOESM4_ESM.tif
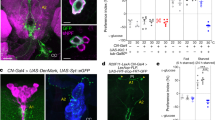
S Fig. 4 Hypomorphic sNPF mutant flies live longer when starved. The hypomorphic sNPF c00448 mutant (sNPF mutant) flies display significantly extended survival at starvation compared to two wild type controls (w 1118 and Oregon; OR) (p < 0.0001; n= 76-108 for the three genotypes, 3 replicates). (TIFF 299 kb)
18_2012_1097_MOESM5_ESM.tif
S Fig. 5 Whole body carbohydrate and lipid levels after corazonin and sNPF knockdown in DLPs. a Whole body trehalose levels (mg/g wet weight) in knockdown flies and parental controls is the same in all genoypes in fed flies. b Change in glycogen levels after 24 h starvation is also the same in all genotypes. c The TAG levels (mg/g wet weight) in normally fed flies do not differ significantly between genotypes. (TIFF 927 kb)
18_2012_1097_MOESM6_ESM.tif
S Fig. 6 Whole body carbohydrate and lipid levels after in sNPF mutant flies and after rescue of sNPF in DLPs (in mutant background), compared to parental mutant flies. In a - c flies were fed normal food. a No difference between genotypes for whole body trehalose levels. b With sNPF rescue in DLPs the glycogen levels are higher than in mutants and controls. (**= p<0,01; Two way ANOVA). c Levels of TAG in fed flies are significantly higher after rescue of sNPF in DLPs (**= p<0.01; Two way ANOVA). (TIFF 535 kb)
Rights and permissions
About this article
Cite this article
Kapan, N., Lushchak, O.V., Luo, J. et al. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell. Mol. Life Sci. 69, 4051–4066 (2012). https://doi.org/10.1007/s00018-012-1097-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1097-z