Abstract
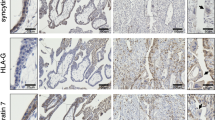
Insulin-like growth factors (IGFs) influence placental cell (cytotrophoblast) kinetics. We recently reported that the protein tyrosine phosphatase (PTP) SHP-2 positively regulates IGF actions in the placenta. In other systems, the closely related PTP, SHP-1, functions as a negative regulator of signaling events but its role in the placenta is still unknown. We examined the hypothesis that SHP-1 negatively regulates IGF actions in the human placenta. Immunohistochemical (IHC) analysis demonstrated that SHP-1 is abundant in cytotrophoblast. SHP-1 expression was decreased in first-trimester placental explants using siRNA; knockdown did not alter IGF-induced proliferation but it significantly enhanced proliferation in serum-free conditions, revealing that placental growth is endogenously regulated. Candidate regulators were determined by using antibody arrays, Western blotting, and IHC to examine the activation status of multiple receptor tyrosine kinases (RTKs) in SHP-1-depleted explants; amongst the alterations observed was enhanced activation of EGFR, suggesting that SHP-1 may interact with EGFR to inhibit proliferation. The EGFR tyrosine kinase inhibitor PD153035 reversed the elevated proliferation seen in the absence of SHP-1. This study demonstrates a role for SHP-1 in human trophoblast turnover and establishes SHP-1 as a negative regulator of EGFR activation. Targeting placental SHP-1 expression may provide therapeutic benefits in common pregnancy conditions with abnormal trophoblast proliferation.





Similar content being viewed by others
References
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7:833–846
Neel BG, Gu H, Pao L (2003) The `Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28:284–293
Ostman A, Bohmer FD (2001) Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol 11:258–266
Forbes K, West G, Garside R, Aplin JD, Westwood M (2009) The protein-tyrosine phosphatase, SRC homology-2 domain containing protein tyrosine phosphatase-2, is a crucial mediator of exogenous insulin-like growth factor signaling to human trophoblast. Endocrinology 150:4744–4754
Forbes K, Westwood M, Baker PN, Aplin JD (2008) Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am J Physiol Cell Physiol 294:C1313–C1322
Norris K, Norris F, Kono DH, Vestergaard H, Pedersen O, Theofilopoulos AN, Moller NP (1997) Expression of protein-tyrosine phosphatases in the major insulin target tissues. FEBS Lett 415:243–248
Adams TE, Epa VC, Garrett TP, Ward CW (2000) Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 57:1050–1093
Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournes B, Faure R, Olivier M, Beauchemin N, Shulman GI, Siminovitch KA, Kim JK et al (2006) The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med 12:549–556
Randhawa R, Cohen P (2005) The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab 86:84–90
Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA (1995) Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J Exp Med 181:2077–2084
Zatelli MC, Piccin D, Tagliati F, Bottoni A, Luchin A (2005) degli Uberti EC. SRC homology-2-containing protein tyrosine phosphatase-1 restrains cell proliferation in human medullary thyroid carcinoma. Endocrinology 146:2692–2698. http://endo.endojournals.org/content/146/6/2692.long
Zhang J, Somani AK, Siminovitch KA (2000) Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol 12:361–378
Duchesne C, Charland S, Asselin C, Nahmias C, Rivard N (2003) Negative regulation of beta-catenin signaling by tyrosine phosphatase SHP-1 in intestinal epithelial cells. J Biol Chem 278:14274–14283
Zapata PD, Ropero RM, Valencia AM, Buscail L, Lopez JI, Martin-Orozco RM, Prieto JC, Angulo J, Susini C, Lopez-Ruiz P, Colas B (2002) Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain-containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab 87:915–926
Tsui HW, Siminovitch KA, de Souza L, Tsui FW (1993) Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet 4:124–129
Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR (1993) Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73:1445–1454
Green MC, Shultz LD (1975) Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered 66:250–258
Kingdom J, Huppertz B, Seaward G, Kaufmann P (2000) Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol 92:35–43
Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D’Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T (2005) Placental phenotypes of intrauterine growth. Pediatr Res 58:827–832
Merviel P, Carbillon L, Challier JC, Rabreau M, Beaufils M, Uzan S (2004) Pathophysiology of preeclampsia: links with implantation disorders. Eur J Obstet Gynecol Reprod Biol 115:134–147
Jansson T, Powell TL (2006) Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor?—a review. Placenta 27:91–97
Aplin JD (2010) Developmental cell biology of human villous trophoblast: current research problems. Int J Dev Biol 54:323–329
Forbes K, Desforges M, Garside R, Aplin JD, Westwood M (2009) Methods for siRNA-mediated reduction of mRNA and protein expression in human placental explants, isolated primary cells and cell lines. Placenta 30:124–129
Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ (1994) A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 265:1093–1095
Cazorla M, Jouvenceau A, Rose C, Guilloux J-P, Pilon C, Dranovsky A, Prémont J (2010) Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice. PLoS One 5:e9777
Forbes K, Farrokhnia F, Aplin JD, Westwood M (2012) Dicer-dependent miRNAs provide an endogenous restraint on cytotrophoblast proliferation. Placenta 33:581–585
Forbes K, Westwood M (2010) Maternal growth factor regulation of human placental development and fetal growth. J Endocrinol 207:1–16
Amemiya K, Kurachi H, Adachi H, Morishige KI, Adachi K, Imai T, Miyake A (1994) Involvement of epidermal growth factor (EGF)/EGF receptor autocrine and paracrine mechanism in human trophoblast cells: functional differentiation in vitro. J Endocrinol 143:291–301
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366:2–16
Forbes K, Souquet B, Garside R, Aplin JD, Westwood M (2010) Transforming growth factor-{beta} (TGF{beta}) receptors I/II differentially regulate TGF{beta}1 and IGF-binding protein-3 mitogenic effects in the human placenta. Endocrinology 151:1723–1731
Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T (2009) Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 150:3774–3782
Johnstone ED, Sibley CP, Lowen B, Guilbert LJ (2005) Epidermal growth factor stimulation of trophoblast differentiation requires MAPK11/14 (p38 MAP kinase) activation. Biol Reprod 73:1282–1288
Amin S, Kumar A, Nilchi L, Wright K, Kozlowski M (2011) Breast cancer cells proliferation is regulated by tyrosine phosphatase SHP1 through c-jun N-terminal kinase and cooperative induction of RFX-1 and AP-4 transcription factors. Mol Cancer Res 9:1112–1125
Simoneau M, Boulanger J, Coulombe G, Renaud MA, Duchesne C, Rivard N (2008) Activation of Cdk2 stimulates proteasome-dependent truncation of tyrosine phosphatase SHP-1 in human proliferating intestinal epithelial cells. J Biol Chem 283:25544–25556
Nakata K, Suzuki Y, Inoue T, Ra C, Yakura H, Mizuno K (2011) Deficiency of SHP1 leads to sustained and increased ERK activation in mast cells, thereby inhibiting IL-3-dependent proliferation and cell death. Mol Immunol 48:472–480
Han VK, Bassett N, Walton J, Challis JR (1996) The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab 81:2680–2693
Krukier II, Pogorelova TN, Orlov VI (2007) Production and reception of growth factors in the placenta during physiological and gestosis complicated pregnancy. Biomed Khim 53:86–90
Dungy LJ, Siddiqi TA, Khan S (1991) Transforming growth factor-beta 1 expression during placental development. Am J Obstet Gynecol 165:853–857
Vuckovic M, Genbacev O, Kumar S (1992) Immunohistochemical localisation of transforming growth factor-beta in first and third trimester human placenta. Pathobiology 60:149–151
Zhong W, Wang QT, Sun T, Wang F, Liu J, Leach R, Johnson A, Puscheck EE, Rappolee DA (2006) FGF ligand family mRNA expression profile for mouse preimplantation embryos, early gestation human placenta, and mouse trophoblast stem cells. Mol Reprod Dev 73:540–550
Anteby EY, Natanson-Yaron S, Hamani Y, Sciaki Y, Goldman-Wohl D, Greenfield C, Ariel I, Yagel S (2005) Fibroblast growth factor-10 and fibroblast growth factor receptors 1–4: expression and peptide localization in human decidua and placenta. Eur J Obstet Gynecol Reprod Biol 119:27–35
Reiter JL, Maihle NJ (2003) Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann N Y Acad Sci 995:39–47
Sun CY, Hu Y, Huang J, Chu ZB, Zhang L, She XM, Chen L (2010) Brain-derived neurotrophic factor induces proliferation, migration, and VEGF secretion in human multiple myeloma cells via activation of MEK-ERK and PI3 K/AKT signaling. Tumour Biol 31:121–128
You Y, Li W, Gong Y, Yin B, Qiang B, Yuan J, Peng X (2010) ShcD interacts with TrkB via its PTB and SH2 domains and regulates BDNF-induced MAPK activation. BMB Rep 43:485–490
Merdek KD, Yang X, Taglienti CA, Shaw LM, Mercurio AM (2007) Intrinsic signaling functions of the beta4 integrin intracellular domain. J Biol Chem 282:30322–30330
Qiu L, Zhou C, Sun Y, Di W, Scheffler E, Healey S, Kouttab N, Chu W, Wan Y (2006) Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. Int J Oncol 29:1003–1011
Mayeur S, Silhol M, Moitrot E, Barbaux S, Breton C, Gabory A, Vaiman D, Dutriez-Casteloot I, Fajardy I, Vambergue A, Tapia-Arancibia L, Bastide B et al (2010) Placental BDNF/TrkB signaling system is modulated by fetal growth disturbances in rat and human. Placenta 31:785–791
Tenev T, Keilhack H, Tomic S, Stoyanov B, Stein-Gerlach M, Lammers R, Krivtsov AV, Ullrich A, Bohmer FD (1997) Both SH2 domains are involved in interaction of SHP-1 with the epidermal growth factor receptor but cannot confer receptor-directed activity to SHP-1/SHP-2 chimera. J Biol Chem 272:5966–5973
Shibasaki Y, Matsubara H, Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E et al (2001) Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension 38:367–372
Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, Bohmer FD (1998) Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J Biol Chem 273:24839–24846
Dackor J, Caron KM, Threadgill DW (2009) Placental and embryonic growth restriction in mice with reduced function epidermal growth factor receptor alleles. Genetics 183:207–218
Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376:337–341
Fondacci C, Alsat E, Gabriel R, Blot P, Nessmann C, Evain-Brion D (1994) Alterations of human placental epidermal growth factor receptor in intrauterine growth retardation. J Clin Invest 93:1149–1155
Fukami T, Yoshizato T, Miyamoto S, Yagi H, Yotsumoto F, Nabeshima K, Hachisuga T, Kuroki M, Kawarabayashi T (2009) Amphiregulin regulates the production of human chorionic gonadotropin in trophoblasts. Life Sci 84:796–804
Li RH, Zhuang LZ (1997) The effects of growth factors on human normal placental cytotrophoblast cell proliferation. Hum Reprod 12:830–834
Lysiak JJ, Han VK, Lala PK (1993) Localization of transforming growth factor alpha in the human placenta and decidua: role in trophoblast growth. Biol Reprod 49:885–894
Lysiak JJ, Johnson GR, Lala PK (1995) Localization of amphiregulin in the human placenta and decidua throughout gestation: role in trophoblast growth. Placenta 16:359–366
Chen Y, Wu XX, Tan JP, Liu ML, Liu YL, Zhang JP (2012) Effects of low molecular weight heparin and heparin-binding epidermal growth factor on human trophoblast in first trimester. Fertil Steril 97:764–770
Li H, Dakour J, Kaufman S, Guilbert LJ, Winkler-Lowen B, Morrish DW (2003) Adrenomedullin is decreased in preeclampsia because of failed response to epidermal growth factor and impaired syncytialization. Hypertension 42:895–900
Sugano M, Tsuchida K, Maeda T, Makino N (2007) SiRNA targeting SHP-1 accelerates angiogenesis in a rat model of hind limb ischemia. Atherosclerosis 191:33–39
Demir R, Seval Y, Huppertz B (2007) Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem 109:257–265
Gu A, Shively JE. Angiopoietins-1 and -2 play opposing roles in endothelial sprouting of embryoid bodies in 3D culture and their receptor Tie-2 associates with the cell–cell adhesion molecule PECAM1. Exp Cell Res 2011
Bhattacharya R, Kwon J, Wang E, Mukherjee P, Mukhopadhyay D (2008) Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration. J Mol Signal 3:8
Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, Gustin JA, Baerwald MR, Jaffe EA, Warren RS, Donner DB (2000) Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem 275:11216–11221
Acknowledgments
This study was funded by a project grant from The Biotechnology and Biological Sciences Research Council, UK (Grant Reference: BBE0076781). LS was funded by a Society for Endocrinology (UK) summer studentship. KF is funded by a University of Manchester Stepping Stone Fellowship and the Maternal and Fetal Health Research Centre is supported by funding from the NIHR Manchester Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forbes, K., Skinner, L., Aplin, J.D. et al. The tyrosine phosphatase SHP-1 negatively regulates cytotrophoblast proliferation in first-trimester human placenta by modulating EGFR activation. Cell. Mol. Life Sci. 69, 4029–4040 (2012). https://doi.org/10.1007/s00018-012-1067-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1067-5