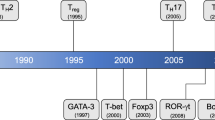
Abstract
Naïve CD4+ T cells undergo massive cell proliferation upon encountering their cognate ligand. This proliferation depends upon appropriate cues from the antigen-presenting cells that have processed the antigen and present the peptide to the T cells, and requires the establishment of a cytokine environment that can support such proliferation. Expansion of antigen-specific CD4+ T cells needs to be coupled with differentiation into one of several effector/regulatory phenotypes if the priming event is to result in cells that can initially act to control the particular pathogen that elicited the response, and later to serve as memory cells to insure an appropriate response upon reintroduction of the pathogen. Here, we discuss the initiation of T helper lineage commitment, the positive feedback regulation by the cytokine environment to enhance and stabilize the differentiation into distinct T helper subsets, and the biological significance of CD4+ T cell plasticity and long-term CD4+ T cell memory.
Similar content being viewed by others
References
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136:2348–2357
Killar L, MacDonald G, West J, Woods A, Bottomly K (1987) Cloned, Ia-restricted T cells that do not produce interleukin 4(IL4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol 138:1674–1679
Hu-Li J, Huang H, Ryan J, Paul WE (1997) In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon γ production is cytokine-dependent. Proc Natl Acad Sci USA 94:3189–3194
Sadick MD, Locksley RM, Tubbs C, Raff HV (1986) Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-γ in response to Leishmania antigens in vitro. J Immunol 136:655–661
Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM (1989) Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct T cell subsets. J Exp Med 169:59–72
Noben-Trauth N, Paul WE, Sacks DL (1999) IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol 162:6132–6140
Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE (1990) Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med 172:921–929
Swain SL, Weinberg AD, English M, Huston G (1990) IL-4 directs the development of Th2-like helper effectors. J Immunol 145:3796–3806
Seder RA, Paul WE, Davis MM, Fazekas de St Groth B (1992) The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med 176:1091–1098
Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM (1992) Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA 89:6065–6069
Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM (1993) Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547–549
Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382:171–174
Kaplan MH, Sun YL, Hoey T, Grusby MJ (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382:174–177
Kaplan MH, Schindler U, Smiley ST, Grusby MJ (1996) Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4:313–319
Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S (1996) Essential role of Stat6 in IL-4 signalling. Nature 380:627–630
Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN (1996) Lack of IL-4-induced Th2 responses and IgE class switching in mice with disrupted Stat6 gene. Nature 380:630–633
Dent AL, Hu-Li J, Paul WE, Staudt LM (1998) T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc Natl Acad Sci USA 95:13823–13828
Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A (2000) Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol 164:3047–3055
Bradley LM, Dalton DK, Croft M (1996) A direct role for IFN-γ in regulation of Th1 cell development. J Immunol 157:1350–1358
Lu B, Ebensperger C, Dembic Z, Wang Y, Kvatyuk M, Lu T, Coffman RL, Pestka S, Rothman PB (1998) Targeted disruption of the interferon-γ receptor 2 gene results in severe immune defects in mice. Proc Natl Acad Sci USA 95:8233–8238
Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669
Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ (2001) T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc Natl Acad Sci USA 98:15137–15142
Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM (2002) T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol 3:549–557
Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE (2004) Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA 101:3880–3885
Yamane H, Zhu J, Paul WE (2005) Independent roles for IL-2 and GATA-3 in stimulating naïve CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med 202:793–804
Kagami S, Nakajima H, Kumano K, Suzuki K, Suto A, Imado K, Davey HW, Saito Y, Takatsu K, Leonard WJ, Iwamoto I (2000) Both Stat5a and Stat5b are required for antigen-induced eosinophil and T-cell recruitment into the tissue. Blood 95:1370–1377
Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ (2008) Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat Immunol 9:1288–1296
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing cells. Immunity 24:179–189
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT (2006) Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231–234
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133
Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C (2007) Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448:480–483
Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK (2007) IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448:484–487
Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR (2007) IL-6 programs TH-17 differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974
Maldonado-López R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M (1999) CD8α+ and CD8α− subclass of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med 189:587–592
Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR (1999) Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA 96:1036–1041
Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F (2004) Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol 5:1260–1265
Yoshimoto T, Paul WE (1994) CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med 179:1285–1295
Seder RA, Paul WE, Dvorak AM, Sharkis SJ, Kagey-Sobotka A, Niv Y, Finkelman FD, Barbieri SA, Galli SJ, Plaut M (1991) Mouse splenic and bone marrow cell populations that express high-affinity Fcε receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci USA 88:2835–2839
Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H (1995) Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature 373:255–257
Perona-Wright G, Mohrs K, Mohrs M (2010) Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat Immunol 11:520–526
van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G (2008) In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci USA 105:12423–12428
Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF Jr, Guo L, Paul WE (2004) Conditional deletion of Gata3 shows its essential function in TH1–TH2 responses. Nat Immunol 5:1157–1165
Pai SY, Truitt ML, Ho IC (2004) GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci USA 101:1993–1998
Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587–596
Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A (2001) A critical role for NF-κB in Gata3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol 2:45–50
Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR (2005) Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J Immunol 175:2102–2110
Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA (2007) Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27:89–99
Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS (2007) Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27:100–110
Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, Sen JM (2009) T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-γ. Nat Immunol 10:992–999
Constant S, Bottomly K (1997) Induction of TH1 and TH2 CD4+ T cell responses: The alternative approaches. Annu Rev Immunol 15:297–322
Yamashita M, Hashimoto K, Kimura M, Kubo M, Tada T, Nakayama T (1998) Requirement for p56lck tyrosine kinase activation in Th subset differentiation. Int Immunol 10:577–591
Yamashita M, Kimura M, Kubo M, Shimizu C, Tada T, Perlmutter RM, Nakayama T (1999) T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci USA 96:1024–1029
Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T (2005) Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem 280:29409–29419
Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K (2009) Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30:155–167
Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT (2009) Late developmental plasticity in the T helper 17 lineage. Immunity 30:92–107
Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C (2008) Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29:44–56
Hegazy AN, Peine M, Helmstetter C, Panse I, Fröhlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Löhning M (2010) Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32:116–128
Selin LK, Nahill SR, Welsh RM (1994) Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 179:1933–1943
Acknowledgments
The work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. We thank Dr. Ryoji Yagi for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yamane, H., Paul, W.E. Memory CD4+ T Cells: fate determination, positive feedback and plasticity. Cell. Mol. Life Sci. 69, 1577–1583 (2012). https://doi.org/10.1007/s00018-012-0966-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-0966-9