Abstract
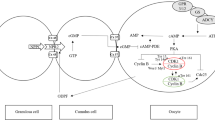
Mammalian oocytes grow and undergo meiosis within ovarian follicles. Fully grown oocytes are arrested at the first meiotic prophase by a mural granulosa origin “arrester” until a surge of luteinizing hormone (LH) from the pituitary at the mid-cycle stimulates the immature oocyte to resume meiosis. Recent evidence indicates that natriuretic peptide precursor type C (NPPC) produced by mural granulosa cells stimulates the generation of cyclic guanosine 3′,5′-monophosphate (cGMP) by cumulus cell natriuretic peptide receptor 2 (NPR2), which diffuses into oocyte via gap junctions and inhibits oocyte phosphodiesterase 3A (PDE3A) activity and cyclic adenosine 3′,5′-monophosphate (cAMP) hydrolysis and maintains meiotic arrest with a high intraoocyte cAMP level. This cAMP is generated through the activity of the Gs G-protein by the G-protein-coupled receptor, GPR3 and GPR12, and adenylyl cyclases (ADCY) endogenous to the oocyte. Further studies suggest that endocrine hormones, such as follicle-stimulating hormone (FSH), LH, 17β-estradiol (E2) and oocyte-derived paracrine factors (ODPFs), participate in oocyte meiosis possibly by the regulation of NPPC and/or NPR2. A detailed investigation of NPPC and NPR2 expression in follicle cells will elucidate the precise molecular mechanisms of gonadotropins, and control the arrest as well as resumption of meiosis.

Similar content being viewed by others
References
Eppig JJ, Vivieros MM, Marin-Bivens C, De La Fuente R (2004) Regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY (eds) The ovary. Elsevier Academic Press, Amsterdam, pp 113–129
Solc P, Schultz RM, Motlik J (2010) Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod 16:654–664
Szybek K (1972) In vitro maturation of oocytes from sexually immature mice. J Endocrinol 54:527–528
Erickson GF, Sorensen RA (1974) In vitro maturation of mouse oocytes isolated from late, middle, and pre-antral Graafian follicles. J Exp Zool 190:123–127
Sorensen RA, Wassarman PM (1976) Relationship between growth and meiotic maturation of mouse oocyte. Dev Biol 50:531–536
Ducibella T (1996) The cortical reaction and development of activation competence in mammalian oocytes. Hum Reprod Update 2:29–42
Mehlmann LM, Mikoshiba K, Kline D (1996) Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol 180:489–498
Ducibella T (1998) Biochemical and cellular insights into the temporal window of normal fertilization. Theriogenology 49:53–65
Dekel N (1995) Molecular control of meiosis. Trends Endocrin Met 6:165–169
Pincus G, Enzmann EV (1935) The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med 62:665–675
Edwards RG (1965) Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208:349–351
Mehlmann LM (2005) Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130:791–799
Tsafriri A, Pomerantz SH (1986) Oocyte maturation inhibitor. Clin Endocrinol Meta 15:157–170
Racowsky C, Baldwin KV (1989) In vitro and in vivo studies reveal that hamster oocyte meiotic arrest is maintained only transiently by follicular-fluid, but persistently by membrana/cumulus granulosa-cell contact. Dev Biol 134:297–306
Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA (2004) The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950
Hinckley M, Vaccari S, Horner K, Chen R, Conti M (2005) The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287:249–261
Richard FJ, Tsafriri A, Conti M (2001) Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 65:1444–1451
Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA (2009) Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869–1878
Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M (2009) Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604
Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ (2010) Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369
Cho WK, Stern S, Biggers JD (1974) Inhibitory effect of dibutyryl camp on mouse oocyte maturation in vitro. J Exp Zool 187:383–386
Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC, Movsesian MA (2005) Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem 280:39168–39174
Törnell J, Billig H, Hillensjo T (1990) Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′, 5′-cyclic-monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand 139:511–517
Törnell J, Carlsson B, Billig H (1990) Atrial natriuretic peptide inhibits spontaneous rat oocyte maturation. Endocrinology 126:1504–1508
Grøndahl C, Breinholt J, Wahl P, Murray A, Hansen TH, Faerge I, Stidsen CE, Raun K, Hegele-Hartung C (2003) Physiology of meiosis-activating sterol: endogenous formation and mode of action. Hum Reprod 18:122–129
Schultz RM, Montgomery RR, Belanoff JR (1983) Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol 97:264–273
Eppig JJ, Ward-Bailey PF, Coleman DL (1985) Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod 33:1041–1049
Zhang M, Ouyang H, Xia G (2009) The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol Hum Reprod 15:399–409
Tripathi A, Kumar KV, Chaube SK (2010) Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol 223:592–600
Anderson E, Albertini DF (1976) Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 71:680
Bornslaeger EA, Schultz RM (1985) Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod 33:698–704
Webb RJ, Marshall F, Swann K, Carroll J (2002) Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase A in mammalian oocytes. Dev Biol 246:441–454
Mehlmann LM, Jones TL, Jaffe LA (2002) Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297:1343–1345
Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M (2003) Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 258:385–396
Mehlmann LM (2005) Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol 288:397–404
Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA (2005) Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol 171:255–265
Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G (2005) Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA 102:8922–8926
DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM (2008) Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod 78:667
Vaccari S, Horner K, Mehlmann LM, Conti M (2008) Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol 316:124–134
Tsafriri A, Chun SY, Zhang R, Hsueh AJW, Conti M (1996) Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 178:393–402
Thomas RE, Armstrong DT, Gilchrist RB (2002) Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol 244:215–225
Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V (2004) Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 114:196–205
Downs SM, Eppig JJ (1986) The role of purines in the maintenance of meiotic arrest in mouse oocytes. Tokai J Exp Clin Med 11:463–469
Reinhardt RR, Chin E, Zhou J, Taira M, Murata T, Manganiello VC, Bondy CA (1995) Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterases. J Clin Invest 95:1528–1538
Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ (1998) Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest 102:532–537
Mayes MA, Sirard MA (2002) Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod 66:180–184
Sasseville M, Côté N, Guillemette C, Richard FJ (2006) New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol 6:47
Hubbard CJ (1980) Ovarian cAMP and cGMP fluctuations in the hamster during the oestrous cycle. J Reprod Fertil 59:351–355
Downs SM, Eppig JJ (1987) Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Biol Reprod 36:431–437
Eppig JJ (1991) Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod 45:824–830
Wang S, Ning G, Chen X, Yang J, Ouyang H, Zhang H, Tai P, Mu X, Zhou B, Zhang M, Xia G (2008) PDE5 modulates oocyte spontaneous maturation via cGMP–cAMP but not cGMP–PKG signaling. Front Biosci 13:7087–7095
Zhang W, Colman RW (2000) Conserved amino acids in metal-binding motifs of PDE3A are involved in substrate and inhibitor binding. Blood 95:3380–3386
Bu S, Xie H, Tao Y, Wang J, Xia G (2004) Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endocrinol 223:85–93
Hanafy KA, Krumenacker JS, Murad F (2001) NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit 7:801–819
Rosenzweig A, Seidman CE (1991) Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem 60:229–255
Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, Imura H (1992) Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130:229–239
Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV (1991) Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252:120–123
Jankowski M, Reis AM, MukaddamDaher S, Dam TV, Farookhi R, Gutkowska J (1997) C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol Reprod 56:59–66
Norris RP, Freudzon L, Freudzon M, Hand AR, Mehlmann LM, Jaffe LA (2007) A G(s)-linked receptor maintains meiotic arrest in mouse oocytes, but luteinizing hormone does not cause meiotic resumption by terminating receptor-G(s) signaling. Dev Biol 310:240–249
Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA (2008) Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238
Tsafriri A, Dekel N, Barami S (1982) The role of oocyte maturation inhibitor in follicular regulation of oocyte maturation. J Reprod Fertil 64:541–551
Ogawa Y, Itoh H, Yoshitake Y, Inoue M, Yoshimasa T, Serikawa T, Nakao K (1994) Molecular cloning and chromosomal assignment of the mouse C-type natriuretic peptide (CNP) gene (Nppc): comparison with the human CNP gene (NPPC). Genomics 24:383–387
Downs SM, Coleman DL, Wardbailey PF, Eppig JJ (1985) Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low-molecular weight fraction of porcine follicular-fluid. Proc Natl Acad Sci USA 82:454–458
Ponderato N, Crotti G, Turini P, Duchi R, Galli C, Lazzari G (2002) Embryonic and foetal development of bovine oocytes treated with a combination of butyrolactone I and roscovitine in an enriched medium prior to IVM and IVF. Mol Reprod Dev 62:513–518
Coy P, Romar R, Payton RR, McCann L, Saxton AM, Edwards JL (2005) Maintenance of meiotic arrest in bovine oocytes using the S-enantiomer of roscovitine: effects on maturation, fertilization and subsequent embryo development in vitro. Reproduction 129:19–26
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17:121–155
Zeleznik AJ (2004) Dynamics of primate follicular growth: a physiological perspective. In: Leung PCK, Adashi EY (eds) The ovary, 2nd edn. Elsevier Academic Press, Amsterdam, pp 45–53
Terranova PF, Rice VM (1997) Review: cytokine involvement in ovarian processes. Am J Reprod Immunol 37:50–63
Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A (2005) Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction 130:147–156
Noubani A, Farookhi R, Gutkowska J (2000) B-type natriuretic peptide receptor expression and activity are hormonally regulated in rat ovarian cells. Endocrinology 141:551–559
Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ (2011) Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology (Epub ahead of print)
Licht P, Gallo AB, Aggarwal BB, Farmer SW, Castelino JB, Papkoff H (1979) Biological and binding activities of equine pituitary gonadotrophins and pregnant mare serum gonadotrophin. J Endocrinol 83:311–322
LaPolt PS, Leung K, Ishimaru R, Tafoya MA, You-hsin Chen J (2003) Roles of cyclic GMP in modulating ovarian functions. Reprod Biomed Online 6:15–23
Gill A, Jamnongjit M, Hammes SR (2004) Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol 18:97–104
Jamnongjit M, Gill A, Hammes SR (2005) Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102:16257–16262
Li M, Ai JS, Xu BZ, Xiong B, Yin S, Lin SL, Hou Y, Chen DY, Schatten H, Sun QY (2008) Testosterone potentially triggers meiotic resumption by activation of intra-oocyte SRC and MAPK in porcine oocytes. Biol Reprod 79:897–905
Motola S, Popliker M, Tsafriri A (2007) Are steroids obligatory mediators of luteinizing hormone/human chorionic gonadotropin-triggered resumption of meiosis in mammals? Endocrinology 148:4458–4465
Tsafriri A, Motola S (2007) Are steroids dispensable for meiotic resumption in mammals? Trends Endocrinol Metab 18:321–327
Diaz FJ, Wigglesworth K, Eppig JJ (2007) Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120:1330–1340
Su YQ, Sugiura K, Eppig JJ (2009) Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 27:32–42
Richards JS, Russell DL, Ochsner S, Espey LL (2002) Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64:69–92
Hunzicker-Dunn M, Mayo K (2006) Gonadotropin signaling in the ovary. In: Neill JD (ed) Knobil and Neill’s physiology of reproduction, 3rd edn. Elsevier/Academic Press, San Diego, pp 547–592
Hashimoto N, Kishimoto T, Nagahama Y (1985) Induction and inhibition of meiotic maturation in follicle-enclosed mouse oocytes by forskolin. Dev Growth Differ 27:709–716
Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M (2007) Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924
Hunzicker-Dunn M (1981) Selective activation of rabbit ovarian protein kinase isozymes in rabbit ovarian follicles and corpora lutea. J Biol Chem 256:12185–12193
Panigone S, Hsieh M, Fu M, Persani L, Conti M (2008) Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936
Eppig JJ, Downs SM (1984) Chemical signals that regulate mammalian oocyte maturation. Biol Reprod 30:1–11
Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N (2006) Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147:2280–2286
Mehlmann LM, Kalinowski RR, Ross LF, Hewlett EL, Jaffe LA (2006) Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol 299:345–355
Hubbard CJ (1986) Cyclic AMP changes in the component cells of Graafian follicles: possible influences on maturation in the follicle-enclosed oocytes of hamsters. Dev Biol 118:343–351
Han SJ, Vaccari S, Nedachi T, Andersen CA, Kovacina KS, Roth RA, Conti M (2006) Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J 25:5716–5725
Eppig JJ, Downs SM (1987) The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation. Dev Biol 119:313–321
Kawamura K, Ye Y, Liang CG, Kawamura N, Gelpke MS, Rauch R, Tanaka T, Hsueh AJW (2009) Paracrine regulation of the resumption of oocyte meiosis by endothelin-1. Dev Biol 327:62–70
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684
Conti M, Hsieh M, Park JY, Su YQ (2006) Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723
Dekel N, Lawrence TS, Gilula NB, Beers WH (1981) Modulation of cell-to-cell communication in the cumulus–oocyte complex and the regulation of oocyte maturation by LH. Dev Biol 86:356–362
Salustri A, Siracusa G (1983) Metabolic coupling, cumulus expansion and meiotic resumption in mouse cumuli oophori cultured in vitro in the presence of FSH or dcAMP, or stimulated in vivo by hCG. J Reprod Fertil 68:335–341
Sherizly I, Galiani D, Dekel N (1988) Regulation of oocyte maturation: communication in the rat cumulus–oocyte complex. Hum Reprod 3:761–766
Motlik J, Fulka J, Flechon JE (1986) Changes in intercellular coupling between pig oocytes and cumulus cells during maturation in vivo and in vitro. J Reprod Fertil 76:31–37
Norris RP, Freudzon M, Nikolaev VO, Jaffe LA (2010) Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 140:655–662
Patwardhan VV, Lanthier A (1984) Cyclic GMP phosphodiesterase and guanylate cyclase activities in rabbit ovaries and the effect of in vivo stimulation with LH. J Endocrinol 101:305–310
Conti M, Kasson BG, Hsueh AJW (1984) Hormonal regulation of 3′, 5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology 114:2361–2368
Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, Mulders S, Terada Y, Hsueh AJ 2011 Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod (Epub ahead of print)
Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T (1991) Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129:3200–3207
Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ (2002) Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143:2221–2232
Potter LR, Hunter T (1998) Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J Biol Chem 273:15533–15539
Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK (2010) Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem 111:521–528
Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A (2002) Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrin 187:153–159
Andersen CB, Roth RA, Conti M (1998) Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J Biol Chem 273:18705–18708
Acknowledgments
This work was supported by National Basic Research Program of China (No. 2012CB944401, 2012CB944701).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, M., Xia, G. Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell. Mol. Life Sci. 69, 1279–1288 (2012). https://doi.org/10.1007/s00018-011-0867-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0867-3